Electrochemical assembly of ZnO architectures via deformation and coalescence of soft colloidal templates in reverse microemulsion
Abstract
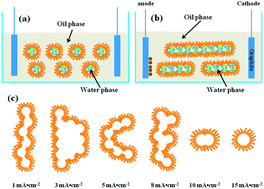
Electrodeposition of ZnO in a novel electrolyte, namely reverse micelles containing zinc nitrate and dissolved oxygen, has been successfully performed. By simply altering the applied current densities or using different substrates, various morphologies are obtained, from 2D ultrathin nanosheets to 3D hierarchical flower-like morphologies, which are quite different from those obtained in conventional electrolytes. Importantly, the behaviors of the soft colloidal templates have a great influence on the morphologies of the ZnO nanostructures. It can be inferred that the versatile deformation, coalescence and rearrangement of the soft colloidal templates also contribute to the specific surface morphologies. The soft colloidal templates are inclined to coalesce and assemble into larger templates on ITO, whereas deformation into different shapes is observed on carbon paper. In this way, an overall new idea to control the shapes and sizes of the template via electrochemistry is proposed.

 Please wait while we load your content...
Please wait while we load your content...