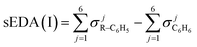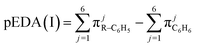On the non-additivity of the substituent effect in ortho-, meta- and para-homo-disubstituted benzenes†
K. Hęclikab,
B. Dębskaa and
J. Cz. Dobrowolski*bc
aRzeszow University of Technology, 12 Powstańcow Warszawy-Street, 35-959 Rzeszow, Poland
bNational Medicines Institute, 30/34 Chełmska-Street, 00-723 Warsaw, Poland. E-mail: j.dobrowolski@nil.gov.pl
cInstitute of Chemistry and Nuclear Technology, 16 Dorodna Street, 03-195 Warsaw, Poland
First published on 1st April 2014
Abstract
The non-additivity of the substituent effect in para-, meta- and ortho-homo-disubstituted benzenes was studied by means of the sEDA(I) and pEDA(I) substituent effect descriptors. The non-additivity effect on σ-valence orbitals is smaller than that on π-ones. For para- and ortho- substitution, the non-additivity effect on π-valence orbitals is ca. 2-times larger than that on σ-ones while for the meta-substitution, it is relatively small and similar in size. In general, there is: (i) the exponential-like increase of the non-additivity on the π-valence ring orbitals with the increase of pEDA(I), i.e., with substituent π-electron-donor properties; (ii) a lack of analogous correlations for the effect on the σ-orbitals; (iii) for para- and ortho-substituted benzenes, but not for meta-isomers, there is a rational-like concave downward decrease of the non-additivity of the σ-effect with an increase of the pEDA(I) descriptor. Thus, the non-additivity increases with the π-electron-donating character of the substituent, while the lack of a similar effect on σ-orbitals is connected to the locality of the σ-donating–accepting substituent effect. The decrease of the non-additivity of the sEDA(I) descriptor as the pEDA(I) descriptor is increased shows the presence of the hyperconjugation effect and the reorganization of the ring σ-electrons with the change of the π-electron systems.
Introduction
Since Hammett's introduction of the substituent effect constants,1 there has been a continuous increase of the importance of this powerful idea in chemistry, medicinal chemistry and materials sciences. Hammett constructed substituent constants based separately on para- and meta-substituted benzoic acids. Thus, already at the beginning he indirectly indicated the complex nature of the substituent effect. Initially the constants were not considered as constituted of different components, however, soon it appeared that the electronegativity,2–8 “the power of an atom in a molecule to attract electrons itself”,2 was related to the substituent effect.9–11 Marriott and Reynolds12 were the first to suggest the substitution effect is combined from field and resonance components but Swain and Lupton13 proposed separating them by a bilinear form with two variable parameters: σF and σR, respectively. Another two components of the substituent effect, electronegativity and polarization, were added by Taft and Topsom in 1987,14 and the linear Hammett expression is, in general, written in the quadrilinear form:| y = y0 + ρFσF + ρRσR + ρασα + ρχσχ + ε |
At first, the substituent effect was mostly studied in substituted benzenes and other aromatic systems. Subsequently, Charton analyzed the effect in acetylenic15 and nonaromatic unsaturated systems.16 Recently, the role of π-electron structure and the substituent effect in nonaromatic molecules were studied more frequently (for example in ethane derivatives,17 ethylenes and acetylenes,18 disubstituted acetylenes,19 and disubstituted diacetylenes20).
In 2009 Ozimiński and Dobrowolski constructed two new descriptors of the substituent effect, sEDA(I) and pEDA(I), based on series of over 30 benzene monoderivatives21 and NBO method of population analysis.22–26 The sEDA(I) and pEDA(I) descriptors express electron shift between a core molecule and a substituent within separated π- and σ-electron systems. They inform about the number of electrons donated to or withdrawn from the core molecule by a substituent. The positive value denotes electron donation to the core molecule and the negative value denotes the withdrawing. The sEDA(I) descriptor was shown to correlate fairly well with the Boyd & Edgecombe27 and Boyd & Boyd28 scales, expressing the inductive (electronegativity) effect, while the pEDA(I) descriptor was shown to correlate well with the Taft-Topsom resonance constant.14,29 The NBO methodology was further used to develop the “second order” sEDA(II) and pEDA(II) descriptors of the heteroatom incorporation effect in five- and six-membered rings.30 Recently, another two new sEDA(=) and pEDA(=) descriptors, expressing substituent effect through a double bond, were constructed based on benzoquinone and cyclopenta-2,4-dienone analogs.31
The sEDA(I) and pEDA(I) descriptors were used to study a variety of problems: aromaticity in fulvene derivatives and their complexes;32–43 stability and tautomerism of 1-deazapurine derivatives,44 azoles, phospholes and phosphodiazoles;45–47 the energetic landscape of an optical molecular switch;48 substituent effects in tetrazole and benzene systems;49 substituent effects in 1,4-disubstituted benzene and cyclohexadiene systems;50 aromaticity of fluorinated pyridines51 and imidazoles;52 in analysis of pharmacologically active hydroxyquinoline derivatives53 and benzodiazepinone systems.54
The effect is additive if and only if the effect of sum is equal to sum of the effects: E(x + y) = E(x) + E(y). Otherwise it is non-additive. Hammett introduced his constants separately for the meta- and para-positions of substituents in benzoic acid with perfect conscious of different reactivity of these two kinds of derivatives.1 Thus, already Hammett indirectly considered the non-additivity of the substituent effects. However, the pseudo-mono-substituted benzoic acids (with invariant –COOH substituent) are hardly adequate to study the additivity effects, because the “second” substituent is in fact the third one. The sEDA(I) and pEDA(I) substitution effect descriptors are constructed based on monoderivatives of benzene and are the appropriate tool for studies of the substituent effect non-additivity.
So far, predominatingly two substituents were considered.55–57 Studies on trisubstituted molecules were rarer.58 So, already the earliest studies had taken into account the non-additivity factor of the Hammett constants. However, the constants that for years determined direction of studies on substituent effects, were inadequate to study the non-additivity problem. Sometimes, the non-additivity problem appeared as a side result of study on quite different topic. For example, an evident non-additivity of the substituent effect was shown by Shahamirian, Cyrański and Krygowski who analyzed ten different substituents in monosubstituted 1,2- and 2,3-naphthoquinones, which may be considered as naphthalenes disubstituted by single and double bond substituents.59 This allowed perceiving that in naphthoquinone derivatives, the substituent effect depended on the path between the single and double bonded substituents and, in particular, depended on whether the number of C-atoms separating the two substitution positions was even or odd.
Important theoretical reasons for non-additivity of substituent effect were given by Gineityte based on perturbational treatment of Hückel hamiltonian for disubstituted benzenes with two substituents of different electron-donor–acceptor characteristic.60 Each substituent produces a perturbation of parent benzene orbitals. It changes, inter alia, the electron occupancy of the parent molecular orbitals. Depend on electron-donor–acceptor character the (different) substituents alter charge in the ring and induce transfer of charge between the substituents. The changes were considered up to fourth order of perturbational scheme. The non-additivities occur as a result of mutual strengthening or quenching of electron occupancies in para-, meta- and ortho-disubstituted benzenes especially at the sites of substitution. The meticulous analysis led the author to the conclusion that the largest intersubstituent interaction may be expected for para- and ortho-substitution whereas the smallest is likely to be peculiar to meta-derivatives of benzene.60
Differences in behavior of para- and meta-homodisubstituted benzene derivatives were studied using descriptors of π-electron delocalization such as aromatic stabilization energies (ASE) and substituent effects stabilization energies (SESE), obtained from the appropriate homodesmotic reactions, as well as NICS and HOMA aromaticity indices.61 This study revealed that, in agreement with Gineityte prediction,62 the π-electron stabilization/destabilization effects for para-homodisubstituted benzenes are much stronger than those for the meta-analogs. Moreover, in all cases the electron donating substituents destabilize the systems. An analogous studies on meta- and para-nitrophenolates led to the conclusion that para-type of systems usually exhibit a stronger variation in any kind of parameters than the meta-type ones.62 Despite of showing and rationalizing differences in para- and meta-homodisubstitution the studies on π-electron delocalization and aromaticity did not uncover clear indication of substituent effect non-additivity.
In monosubstituted compounds, the global size and structure of substituent are less important than the substituent effect. In di- or multisubstituted compounds, the steric effect is another important intramolecular effect that may be a main source of non-additivity of the substituent effect. The effect is mainly governed by repulsive overlap between closed-shell orbitals of the substituent and, in a certain part, by attractive dispersive interactions.63,64 The steric effect was infrequently studied in close combination with the substituent effect.65 In the case of close vicinity of substituents in di- or multisubstituted systems, as in hexabromobenzene or octabromonaphthalene66 or diisopropylobenzenes or naphthalenes67,68 the steric effects may influence molecular structure much more than the sole substituent.
The intermolecular factors such as hydrogen bonding formed by substituents with compounds surrounding the substituted molecule, or simply a solvent, may dramatically change the electron distribution of the substituted core.69–72 The same holds true for the electron donor–acceptor interactions occurring between the electron-deficient and electron-rich molecules which are omnipresent in electron transfer,73–75 central ion interactions, stacking interactions between nucleic base pairs,76–79 and in combination with H-bonding, shape the peptide conformations due to the interactions of aromatic moieties of the amino acids.80,81 The two kinds of intermolecular interactions in specific, e.g., protic, solvents give rise to complex dissociation and kinetic phenomena producing, inter alia, transformation of neutral substituents into charged ones. Hydrophobicity/lipophilicity of the medium, being expression of the dispersive interaction of a molecule and its environment, is yet another factor influencing, for example, conformation of the substituents changing electron distribution inside the core molecule. Last but not least, it is important that the para- homodisubstituted benzenes exhibit no dipole moment whereas meta- and ortho-substituted benzenes are polar and the dipole moment of the ortho- is usually larger than their meta-isomers. The polarity determines not only intermolecular behavior of the molecules but also intramolecular distribution of molecular charge.
The aim of this study is three-fold. (i) To quantitatively determine the non-additivity of the substituent effect in homo-disubstituted benzenes. (ii) To evaluate role of the substitution para-, meta- and ortho-positions on the non-additivity. (iii) To estimate the additivity separately on the σ- and π-valence orbitals.
Methods
All of the essential calculations were performed using the hybrid Becke three-parameter Lee–Yang–Parr DFT B3LYP functional,82,83 for which the reliability of calculations of the ground state geometries has been widely assessed.84 The correlation consistent aug-cc-pVDZ Dunning85,86 basis set was employed as well as 6-31G(d,p)87 and 6-311++G(d,p)88 basis set were used. Each minimum was confirmed by the positive harmonic frequencies.89 The complete analysis was made for global minimum energy structures chosen for calculations of several conformations of each model structure. All the calculations were performed using the Gaussian 09 software.90 Natural population analysis (NPA), based on the natural atomic orbitals (NAOs) of the natural bond orbital (NBO) theory91–93 (NBO Version 3.1 (ref. 94) as implemented in Gaussian 09), was used to reveal the σ and π electron shift between the core molecule and the double bonded substituent.95The sEDA and pEDA descriptors for monosubstituted benzene were constructed by Ozimiński and Dobrowolski21 according to following equations:
where σj and πj denote sums of occupancies of all atomic orbitals of the j-th benzene ring C-atom contributing to the valence π- and σ-molecular orbitals in the molecule indexed by i. The σ-effect is defined as the sum of occupancies of s, px and py valence orbitals of all the ring C atoms (where xy is the ring plane) and π-effect is defined by the sum of occupancies of the pz orbitals of all the ring C atoms contributing to the benzene π electron system. In the case of monosubstituted benzenes by R substituent the right subscript denotes the R–C6H5 molecule, whereas in disubstituted benzenes the R1–C6H4–R2 molecules. For clarity we denote the appropriate descriptors by sEDA(I)[R], pEDA(I)[R] and sEDA(I)[R1 + R2] and pEDA(I)[R1 + R2].
Values of s, px, py and pz populations from over 300 output files were automatically read from *.log or *.out Gaussian file and properly summed by using EDA-Reader application.96 Thanks to our own program, time of read-out necessary parameters values of whole molecule (the energies, dipole-moments, etc.) or selected atoms in molecule (e.g. s, px, py and pz populations) is minimized to couple of seconds.
Results and discussion
Fourteen substituents considered in this study (Table 1) were selected to span the effect on both π- and σ-molecular orbitals of the disubstituted benzene molecule. Indeed, according to the values of the sEDA(I) and pEDA(I) descriptors21 they cover the sEDA(I) scale from σ-donating Li (sEDA(I) = 0.443), BF2 (sEDA(I) = 0.200), and BH2 (sEDA(I) = 0.153), through moderately σ-electron withdrawing SH and CN (sEDA(I) = −0.118 and −0.137), to strongly σ-withdrawing OH (sEDA(I) = −0.623) and F (sEDA(I) = −0.747). Simultaneously, they cover the pEDA(I) scale from π-donating NMe2 and OH (pEDA(I) = 0.174 and 0.121), through π-neutral tBu (pEDA(I) = 0.008), to π-electron withdrawing CHO and BH2 (pEDA(I) = −0.087 and −0.142). Notice, that the sEDA(I) spans much larger scale of over 1 e than pEDA(I) descriptor which extends across ca. 0.3 e. Here the attention is focused on homo-disubstituted benzenes to select the main non-additivity effects and make background for the eventual future analysis in which every pair of the 15 substituents would be considered. However, in such a study 225 systems should be calculated by using more than one basis set. Moreover, some of the substituents can adopt more than one conformation and the number of conformations of disubstituted systems substantially increases. Such a task has been beyond this pilot study. The original sEDA(I) and pEDA(I) descriptors were obtained using the 6-31G(d,p) basis set.21 This basis set is known to yield quite correct values and by its relatively small size guarantees that the set of sEDA(I) and pEDA(I) descriptors can be reproduced and/or extended in most of labs. However, we expected that the non-additivity effects may be subtle and in looking for well validated results we recalculated the systems also with the larger Pople-type 6-311++G(d,p) basis set and the correlation consistent aug-cc-pVDZ Dunning basis set yielding quite satisfactory results. The three basis sets provided sEDA(I) and pEDA(I) values (Table 1) linearly correlating with the regression coefficients exceeding 0.997. However, the intercepts are always slightly lower for correlations between 6-31G(d,p) and aug-cc-pVDZ than between 6-31G(d,p) and 6-311++G(d,p) indicating that the values obtained with 6-31G(d,p) and aug-cc-pVDZ basis sets are closer than those obtained with 6-311++G(d,p) and aug-cc-pVDZ. Remark, also that the CPU times for the 6-311++G(d,p) basis set were 3–4 times greater than those for the 6-31G(d,p) one, while for the aug-cc-pVDZ basis set they were 6–8 times greater. Assuming that the results obtained with the largest aug-cc-pVDZ basis set are the most reliable, the small 6-31G(d,p) basis set better describes the sEDA(I) and pEDA(I) values than the much larger 6-311++G(d,p) one. This is important for further studies of much larger systems. Hereafter, we analyse results yielded by the B3LYP/aug-cc-pVDZ calculations.| Substituents | 6-31G(d,p) | 6-311++G(d,p) | aug-cc-pVDZ | Substituents | 6-31G(d,p) | 6-311++G(d,p) | aug-cc-pVDZ | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| sEDA | pEDA | sEDA | pEDA | sEDA | pEDA | sEDA | pEDA | sEDA | pEDA | sEDA | pEDA | ||
| mono- | ortho- | ||||||||||||
| –BF2 | 0.1928 | −0.0767 | 0.1901 | −0.0761 | 0.2119 | −0.0776 | –BF2, o- | 0.3611 | −0.1188 | 0.3595 | −0.1168 | 0.4002 | −0.1197 |
| –BH2 | 0.1726 | −0.1421 | 0.1928 | −0.1341 | 0.1927 | −0.1359 | –BH2, o- | 0.3326 | −0.2562 | 0.3781 | −0.2397 | 0.3752 | −0.2441 |
| –Br | −0.1964 | 0.0564 | −0.1563 | 0.0579 | −0.1892 | 0.0569 | –Br, o- | −0.3789 | 0.1282 | −0.2961 | 0.1302 | −0.3595 | 0.1284 |
| –CHO | −0.1021 | −0.0875 | −0.0755 | −0.0867 | −0.0946 | −0.0883 | –CHO, o- | −0.2142 | −0.1474 | −0.1608 | −0.1448 | −0.1994 | −0.1489 |
| –Cl | −0.2645 | 0.0627 | −0.2267 | 0.0655 | −0.2656 | 0.0636 | –Cl, o- | −0.5202 | 0.1395 | −0.4442 | 0.1456 | −0.5188 | 0.1420 |
| –CN | −0.1590 | −0.0353 | −0.1282 | −0.0346 | −0.1557 | −0.0348 | –CN, o- | −0.3389 | −0.0451 | −0.2710 | −0.0434 | −0.3265 | −0.0447 |
| –COOH | −0.1101 | −0.0680 | −0.0895 | −0.0674 | −0.1125 | −0.0689 | –COOH, o- | −0.2726 | −0.0761 | −0.2315 | −0.0727 | −0.2806 | −0.0748 |
| –F | −0.6213 | 0.0783 | −0.5834 | 0.0681 | −0.6255 | 0.0684 | –F, o- | −1.2485 | 0.1547 | −1.1756 | 0.1384 | −1.2569 | 0.1385 |
| –Li | 0.4602 | −0.0200 | 0.5493 | −0.0110 | 0.5191 | −0.0111 | –Li, o- | nc | nc | nc | nc | nc | nc |
| –N(CH3)2 | −0.4758 | 0.1741 | −0.4421 | 0.1728 | −0.4817 | 0.1764 | –N(CH3)2, o- | −0.8724 | 0.1751 | −0.8168 | 0.1746 | −0.8965 | 0.1795 |
| –NH2 | −0.4521 | 0.1452 | −0.4103 | 0.1408 | −0.4469 | 0.1407 | –NH2, o- | −0.8777 | 0.2321 | −0.7945 | 0.2227 | −0.8656 | 0.2240 |
| –OCH3 | −0.5613 | 0.1215 | −0.5253 | 0.1178 | −0.5659 | 0.1195 | –OCH3, o- | −1.1223 | 0.2312 | −1.0518 | 0.2279 | −1.1329 | 0.2361 |
| –OH | −0.5614 | 0.1214 | −0.5176 | 0.1126 | −0.5585 | 0.1136 | –OH, o- | −1.1152 | 0.2247 | −1.0336 | 0.2109 | −1.1114 | 0.2127 |
| –SH | −0.1491 | 0.0932 | −0.1200 | 0.0988 | −0.1434 | 0.0958 | –SH, o- | −0.2620 | 0.1385 | −0.2051 | 0.1488 | −0.2585 | 0.1549 |
| –tBu | −0.2402 | 0.0083 | −0.2003 | 0.0095 | −0.2336 | 0.0079 | –tBu, o- | −0.4772 | 0.0110 | nc | nc | −0.4715 | 0.0088 |
| meta- | para- | ||||||||||||
| –BF2, m- | 0.3787 | −0.1445 | 0.3742 | −0.1419 | 0.4178 | −0.1449 | –BF2, p- | 0.3756 | −0.1356 | 0.3713 | −0.1321 | 0.4154 | −0.1347 |
| –BH2, m- | 0.3385 | −0.2713 | 0.3857 | −0.2567 | 0.3846 | −0.2600 | –BH2, p- | 0.3323 | −0.2499 | 0.3769 | −0.2368 | 0.3773 | −0.2401 |
| –Br, m- | −0.3871 | 0.1194 | −0.3064 | 0.1222 | −0.3707 | 0.1204 | –Br, p- | −0.3872 | 0.1176 | −0.3067 | 0.1193 | −0.3715 | 0.1174 |
| –CHO, m– | −0.2113 | −0.1591 | −0.1532 | −0.1574 | −0.1916 | −0.1609 | –CHO, p- | −0.2155 | −0.1489 | −0.1586 | −0.1464 | −0.1970 | −0.1496 |
| –Cl, m- | −0.5260 | 0.1319 | −0.4506 | 0.1376 | −0.5268 | 0.1337 | –Cl, p- | −0.5259 | 0.1291 | −0.4497 | 0.1331 | −0.5270 | 0.1293 |
| –CN, m- | −0.3298 | −0.0537 | −0.2649 | −0.0524 | −0.3203 | −0.0531 | –CN, p- | −0.3319 | −0.0475 | −0.2667 | −0.0466 | −0.3220 | −0.0473 |
| –COOH, m- | −0.2281 | −0.1241 | −0.1829 | −0.1225 | −0.2280 | −0.1267 | –COOH, p- | −0.2300 | −0.1168 | −0.1846 | −0.1149 | −0.2303 | −0.1178 |
| –F, m- | −1.2486 | 0.1614 | −1.1708 | 0.1414 | −1.2557 | 0.1417 | –F, p- | −1.2447 | 0.1519 | −1.1675 | 0.1327 | −1.2521 | 0.1329 |
| –Li, m- | 0.8785 | −0.0534 | 1.0818 | −0.0279 | 1.0231 | −0.0279 | –Li, p- | 0.8995 | −0.0545 | 1.0923 | −0.0311 | 1.0306 | −0.0304 |
| –N(CH3)2, m- | −0.9449 | 0.3351 | −0.8762 | 0.3316 | −0.9603 | 0.3420 | –N(CH3)2, p- | −0.9172 | 0.2676 | −0.8545 | 0.2629 | −0.9384 | 0.2810 |
| –NH2, m- | −0.9009 | 0.2855 | −0.8177 | 0.2786 | −0.8912 | 0.2792 | –NH2, p- | −0.8850 | 0.2456 | −0.8036 | 0.2363 | −0.8756 | 0.2358 |
| –OCH3, m- | −1.1235 | 0.2437 | −1.0499 | 0.2363 | −1.1323 | 0.2396 | –OCH3, p- | −1.1150 | 0.2229 | −1.0438 | 0.2150 | −1.1244 | 0.2179 |
| –OH, m- | −1.1245 | 0.2459 | −1.0368 | 0.2295 | −1.1186 | 0.2316 | –OH, p- | −1.1159 | 0.2233 | −1.0293 | 0.2067 | −1.1105 | 0.2085 |
| –SH, m- | −0.2975 | 0.1916 | −0.2385 | 0.2032 | −0.2850 | 0.1977 | –SH, p- | −0.2955 | 0.1836 | −0.2350 | 0.1871 | −0.2838 | 0.1864 |
| –tBu, m- | −0.4802 | 0.0153 | −0.3870 | 0.0420 | −0.4696 | 0.0132 | –tBu, p- | −0.4800 | 0.0150 | nc | nc | −0.4700 | 0.0134 |
Definition of the non-additivity of the substituent effect
The mathematical sense of non-additive effect is clear: we say that the effect is non-additive if and only if the effect of sum is different from the sum of the effects. Let us denote non-additivity of the descriptor by NA or na with subscript defining the descriptor under consideration and superscript defining the position of substituents in the analyzed system. The substituents under consideration are denoted by R1 or R2 in brackets [R1] or [R2] and the position of substituents, pos, is denoted by letters p, m and o in right superscript of the bracket. Non-additivity, NA, can be expressed as an absolute effect in e units (1) or as a relative value, na, referred to the sum of effects of single substituents expressed in percentage units (2).| NApossEDA = sEDA(I)[R1] + sEDA(I)[R2] − sEDA(I)[R1 + R2]pos | (1a) |
| NApospEDA = pEDA(I)[R1] + pEDA(I)[R2] − pEDA(I)[R1 + R2]pos | (1b) |
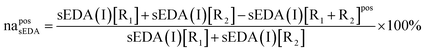 | (2a) |
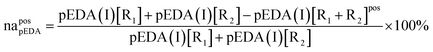 | (2b) |
Most definitions of quantitative criteria are somehow arbitrary. Here we try to rationalize our criteria of non-additivity as follows. We found that our calculations using the aug-cc-pVDZ and 6-31G(d,p) basis sets are in well agreement. Nevertheless, they sometimes differ, but usually by not more than 0.02 e. Therefore, we assumed that if the non-additivity effect is equal to or greater than 0.02 e we can reliably say that criterion of the absolute non-additivity, NA, is satisfied. However, for some systems, the parent substituent effect is small, and thus the eventual non-additivity cannot be large in absolute values. In such cases we use the relative non-additivity, na, criterion which is assuming that the effect is relatively non-additive if it is equal to or greater than 8% of the original.
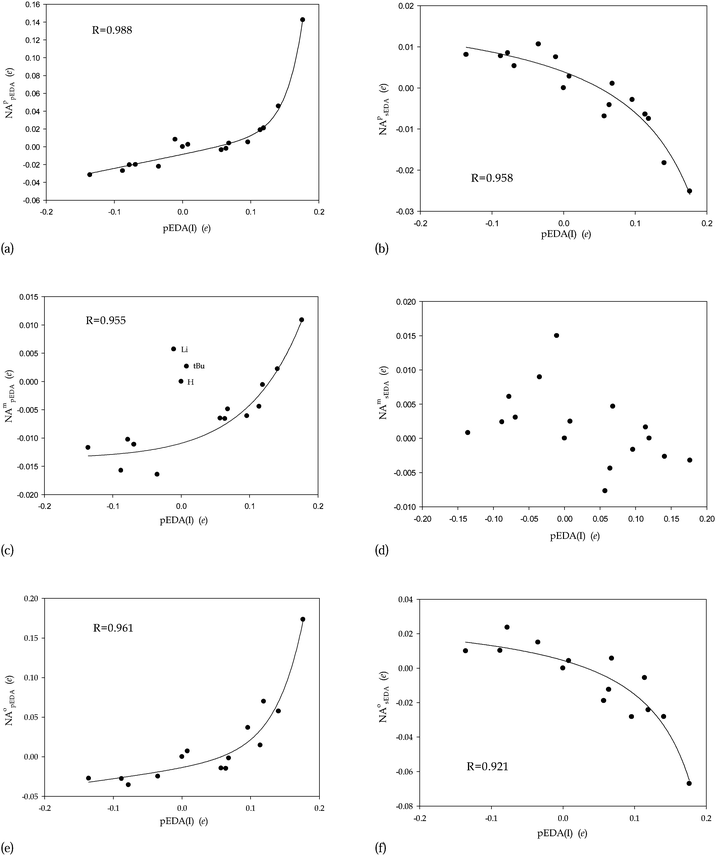
The two types of the EDA descriptors allow distinguishing between two separate components of the substituent effect: the effect on σ and π valence orbitals of the benzene core. The former can be measured by the sEDA(I) descriptor, practically expressing group electronegativity, which is has a short-range influence.21 This is why we supposed that the substituent effect on the benzene σ valence orbitals is relatively additive. On the contrary, the effect on π valence orbitals, measured in terms of pEDA(I) values, correlating with resonance parameters, is propagated through the π-electron system over the whole molecule.21 This is why we expected some noteworthy differences between meta- and para-substitution and the effect of non-additivity. Tables 1–4 demonstrate that our intuition was correct. Before going into details, observe that in general the non-additivity is much more pronounced for pEDA(I) than sEDA(I) descriptor (Tables 1–4, Fig. 1). Indeed, for para-substitution, approximately, NApsEDA 0(−0.03, 0.01) while NAppEDA 0(−0.03, 0.07); for meta-substitution NAmsEDA 0(−0.01, 0.02) while NAmpEDA 0(−0.02, 0.01); and for ortho-substitution the NAosEDA 0(−0.07, 0.06) and NAopEDA 0(−0.06, 0.17). Thus, the non-additivity of sEDA(I) covers 0.04, 0.03, and 0.13 e interval for para-, meta- and ortho-substitution, respectively, whereas, for the analogous substitutions, the non-additivity of pEDA(I) covers 0.1, 0.03 and 0.23 e intervals.
The length of these intervals also demonstrates that the non-additivity of para- is larger than that of meta-substitution, and that the non-additivity of ortho- is much is larger than that of para-substitution.
The origin of the non-additivity of the sEDA(I) and pEDA(I) descriptors in para- and meta-substitution is quite different from that in ortho-substitution. The substituents in meta- and para-positions cannot directly interact with each other through space thus the interaction occurs first and foremost through the σ and π valence orbitals of the benzene core. On the contrary, the substituents in ortho-position are so close that already inter-substituent interaction of simple F-, Cl- and Br-groups can be detected (Table 4). The non-additivity of the ortho-substitution requires comments on direct interactions of the substituents, therefore, it is discussed at the end of this section.
para-Substitution
For the sEDA(I) descriptor, the absolute non-additivity criterion is satisfied only for the N(CH3)2 substituent (Table 2), which is not the strongest σ electron withdrawing group since: sEDA(I)[N(CH3)2] = −0.48 whereas sEDA(I)[F] = −0.62 (Table 1). The relative non-additivity criterion for the sEDA(I) descriptor is not satisfied at all (Table 2). The absolute non-additivity of the pEDA(I) descriptor in para-substituted benzenes occurs for BF2, BH2, CHO, COOH, CN, NH2, N(CH3)2 and OCH3 disubstituted systems (Table 2). The relative non-additivity of the pEDA(I) descriptor is additionally satisfied for Li, tBu and OH. This means that according to the relative non-additivity criterion, only SH, Br, Cl and F substituents exhibit the additive effect.| Homo-disubstitution in para-position | NApsEDA (e) | NAppEDA (e) | napsEDA (%) | nappEDA (%) |
|---|---|---|---|---|
| BF2 | 0.008 | 0.020 | 2 | 13 |
| BH2 | 0.008 | 0.032 | 2 | 12 |
| Br | 0.007 | 0.004 | 2 | 3 |
| CHO | 0.008 | 0.027 | 4 | 15 |
| Cl | 0.004 | 0.002 | 1 | 2 |
| CN | 0.011 | 0.022 | 3 | 32 |
| COOH | 0.005 | 0.020 | 2 | 15 |
| F | 0.001 | 0.004 | 0 | 3 |
| Li | 0.008 | 0.008 | 1 | 37 |
| N(CH3)2 | 0.025 | 0.072 | 3 | 20 |
| NH2 | 0.018 | 0.046 | 2 | 16 |
| OCH3 | 0.007 | 0.021 | 1 | 9 |
| OH | 0.006 | 0.019 | 1 | 8 |
| SH | 0.003 | 0.005 | 1 | 3 |
| tBu | 0.003 | 0.002 | 1 | 16 |
To better understand the origin of the non-additivity, the NApsEDA and NAppEDA absolute non-additivity values were plotted against values of the corresponding sEDA(I) and pEDA(I) descriptors (Fig. 1). First, there is no correlation between the non-additivity values and the sEDA(I) descriptor whereas there are significant non-linear correlations between NApsEDA and NAppEDA values and the pEDA(I) descriptor (Fig. 1a and b). The lack of regular tendency of non-additivities with sEDA(I) denotes that even strong local influence on the σ valence electron system does not significantly perturb the effect in para-position. On the other hand, the monotonic change of the non-additivity with the pEDA(I) descriptor denotes good communication between para-substituents through the π-valence electrons. This is quite expected effect for the NAppEDA non-additivity (Fig. 1a). Indeed, the stronger the π-valence electrons are perturbed by one substituent the stronger is the effect in the para-position because it is just propagated through the π-valence electrons. However, the tendency in Fig. 1b demonstrates that the stronger the π-valence electrons are perturbed by one substituent the stronger the perturbation of the σ valence electron system is propagated to the σ valence electrons in para-position. The latter was not expected and indicates that redistribution of the π-valence electrons influences the redistribution of the σ-valence electrons. This effect clearly show that the stronger π-electron donor is the substituent the larger role in substituent-benzene interaction plays the hyperconjugation effect.
meta-Substitution
For the sEDA(I) descriptor, the absolute non-additivity criterion is satisfied only for Li substituent (Table 3), which is the strongest σ electron donating group: sEDA(I)[Li] = 0.460 (Table 1). However, the relative non-additivity criterion for sEDA(I) descriptor is not satisfied at all (Table 3). Also, the absolute non-additivity of the pEDA(I) descriptor in meta-substituted benzenes is not satisfied (Table 3). Nevertheless, the relative non-additivity of the pEDA(I) descriptor is satisfied for Li, CHO, COOH, CN and tBu. Thus for the most substituents positioned in meta-position the substituent effect is both absolutely and relatively additive. Again, to look deeper insight the substituent non-additivity NAmsEDA and NAmpEDA were plotted against corresponding pEDA(I) values (Fig. 1c and d). Again, the non-linear correlations between NAmpEDA and pEDA(I) descriptor is significant (Fig. 1c). This means that the stronger is the effect in meta-position the more it is propagated to the meta-position through the π-valence electrons. However, there is no correlation between NAmsEDA and pEDA(I) descriptor (Fig. 1d) which was observed before (Fig. 1b). As before, there is no correlation between the non-additivity values and the sEDA(I) descriptor (the lack of correlations is not presented). Observe that quick increase of NApEDA as pEDA(I) is increased in meta- and para-homo-disubstituted benzene derivatives is similar (Fig. 1a and c). Remark that three points excluded from the regression (Fig. 1c) correspond to Li, H and tBu substituents which practically do not contribute to the π-electron system of the benzene core. The lack of regular tendency of non-additivities with sEDA(I) has the same origin as for para-substitution: this is a result of locality of the substituent effect on the σ-valence orbitals of benzene. Thus in this case the hyperconjugation effect has not chance to be revealed. This time, the lack of regular trend in plot of NAmsEDA vs. pEDA(I) is a result of too weak changes generated by disubstitution in the meta-position.| Homo-disubstitution in meta-position | NAmsEDA (e) | NAmpEDA (e) | namsEDA (%) | nampEDA (%) |
|---|---|---|---|---|
| BF2 | 0.006 | 0.010 | 1 | 7 |
| BH2 | 0.001 | 0.012 | 0 | 4 |
| Br | 0.008 | 0.007 | 2 | 6 |
| CHO | 0.002 | 0.016 | 1 | 9 |
| Cl | 0.004 | 0.007 | 1 | 5 |
| CN | 0.009 | 0.016 | 3 | 24 |
| COOH | 0.003 | 0.011 | 1 | 8 |
| F | 0.005 | 0.005 | 0 | 4 |
| Li | 0.015 | 0.006 | 1 | 26 |
| N(CH3)2 | 0.003 | 0.011 | 0 | 3 |
| NH2 | 0.003 | 0.002 | 0 | 1 |
| OCH3 | 0.000 | 0.001 | 0 | 0 |
| OH | 0.002 | 0.004 | 0 | 2 |
| SH | 0.002 | 0.006 | 1 | 3 |
| tBu | 0.002 | 0.003 | 1 | 17 |
ortho-Substitution
The ortho-substitution is specific because the sheer substituent effect is knotted with the inter-substituent interactions. In the case of substituents able to form hydrogen bond, the inter-substituent interactions are expected to be strong even if similar interaction of free molecules is not necessary strong. This is because the substituents in the ortho-position are rigid and are forced to interact. On the other hand, the aprotic substituents exhibiting free electron pairs in the ortho-position repulse each other. Again, rigidity of the substitution increases the effect. The same is true for bulky substituents which in the ortho-position exhibit significant steric repulsion.Complexity of the substituent interaction in the ortho-position is the main reason why this substitution is mainly studied in context of intramolecular hydrogen bonding or the other intramolecular interactions. In fact, when planning the current study, we expected that the effect of inter-substituent interactions of the ortho-positioned substituents will perturb the sheer substituent effect so much, that the conclusive results cannot be obtained. Moreover, studies of the ortho-substitution are more complicated than study of the other disubstitutions of the benzene core because different conformations of substituents do really matter. For example, it is quite important whether two CHO groups are directed to each other by two H-atoms, two O = atoms or form intramolecular O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–H⋯O
C–H⋯O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–H hydrogen bond. The bulky substituents like tBu or N(CH3)2 may also be directed towards each other in several ways.
C–H hydrogen bond. The bulky substituents like tBu or N(CH3)2 may also be directed towards each other in several ways.
As expected, the non-additivity of the disubstitution in the ortho-position is the most pronounced (Table 4). For the sEDA(I) descriptor, the absolute non-additivity criterion is satisfied by five substituents: BF2, COOH, NH2, N(CH3)2 and OCH3 (Table 4). However, the relative criterion for non-additivity is satisfied only for COOH which forms strong OH⋯O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C hydrogen bond. For the stronger σ electron donating substituent, Li, (sEDA(I)[Li] = 0.460, Table 1), the ortho-disubstituted benzene derivative converges to two separate molecules. On the other hand, for the pEDA(I) descriptor, the absolute non-additivity criterion is satisfied by eight substituents: the five which produce the sEDA(I) non-additivity, and BH2, CHO and CN (Table 4). However, the relative non-additivity criterion for pEDA(I) descriptor is not satisfied only for SH, OH and F (Table 4). For OH, which forms quite significant intramolecular H-bond, this is quite surprising.
C hydrogen bond. For the stronger σ electron donating substituent, Li, (sEDA(I)[Li] = 0.460, Table 1), the ortho-disubstituted benzene derivative converges to two separate molecules. On the other hand, for the pEDA(I) descriptor, the absolute non-additivity criterion is satisfied by eight substituents: the five which produce the sEDA(I) non-additivity, and BH2, CHO and CN (Table 4). However, the relative non-additivity criterion for pEDA(I) descriptor is not satisfied only for SH, OH and F (Table 4). For OH, which forms quite significant intramolecular H-bond, this is quite surprising.
| Homo-disubstitution in ortho-position | NAosEDA (e) | NAopEDA (e) | naosEDA (%) | naopEDA (%) |
|---|---|---|---|---|
| BF2 | 0.024 | 0.035 | 6 | 23 |
| BH2 | 0.010 | 0.028 | 3 | 10 |
| Br | 0.019 | 0.015 | 5 | 13 |
| CHO | 0.010 | 0.028 | 5 | 16 |
| Cl | 0.012 | 0.015 | 2 | 12 |
| CN | 0.015 | 0.025 | 5 | 36 |
| COOH | 0.056 | 0.063 | 25 | 46 |
| F | 0.006 | 0.002 | 0 | 1 |
| Li | nc | nc | nc | nc |
| N(CH3)2 | 0.067 | 0.173 | 7 | 49 |
| NH2 | 0.028 | 0.057 | 3 | 20 |
| OCH3 | 0.001 | 0.003 | 0 | 1 |
| OH | 0.006 | 0.015 | 0 | 6 |
| SH | 0.028 | 0.037 | 10 | 19 |
| tBu | 0.004 | 0.007 | 1 | 45 |
The plots of NAosEDA and NAopEDA vs. sEDA(I) and pEDA(I) descriptors are similar to those found for para-substitution (Fig. 1e and f). They exhibit no correlations when plotted against sEDA(I) whereas there are significant non-linear correlations with the pEDA(I) descriptor. The latter effect is quite surprising because it indicates that, except for COOH, the sheer substituent effect of ortho-disubstituted benzene has much greater influence on the non-additivity than the intramolecular interactions. Moreover, the kind of tendencies is similar: NAopEDA increases and NAosEDA decreases non-linearly with pEDA(I) (Fig. 1e and f). Again, the hyperconjugation participates in the substituent effect through one bond but the tendency is a bit more scattered (R = 0.882).
Conclusions
The sEDA(I) and pEDA(I) descriptors used in this study were constructed based on monosubstituted benzenes and enabled for clear definition and straightforward study of the non-additivity of substituent effect in the case of benzene double substitution. Several other descriptors, including original Hammett σp and σm constants, are based on disubstituted benzene derivatives and thus give no chance for systematic and methodologically correct study of the non-additivity of substituent effect in disubstituted systems. The theoretical reasons for non-additivity of the substituent effect were given by Gineityte60 and based on analysis of molecular orbital occupancies. The analysis led to the conclusion that the largest intersubstituent interaction may be expected for para- and ortho- substitution whereas they are the smallest for meta-derivatives of benzene.60This study demonstrates that for some substituents, the substituent effect in disubstituted benzene molecules is non-additive. Moreover, the non-additivity of the substituent effect on σ-valence orbitals is much smaller than that on π-ones. For para- and ortho-substitution, the non-additivity of the substituent effect on π-valence orbitals is ca. 2-times larger than that on σ-valence orbitals while for the meta-benzene derivatives, the non-additivity is relatively small and of the same order for σ- and π-valence orbitals.
The important features of non-additivity of the substituent effect were demonstrated by plots of the absolute non-additivity values against values of the corresponding sEDA(I) and pEDA(I) values. For all three homo-disubstitutions: (i) the monotonic, exponential-like, increase of the non-additivity of the pEDA(I) descriptor of disubstituted systems with increase of the pEDA(I) descriptor of monosubstituted reference is observed; (ii) there is no correlation between the non-additivity values and sEDA(I) descriptor of disubstituted systems with increase of the sEDA(I) descriptor of monosubstituted reference. For para- and ortho-homo-disubstituted benzenes, but not for meta-homo-disubstitution (iii) there is the monotonic, rational-like concave downward, decrease of the non-additivity of the sEDA(I) descriptor of disubstituted systems with increase of the pEDA(I) descriptor of the monosubstituted reference.
The exponential-like increase of the pEDA(I) descriptor as the pEDA(I) descriptor denotes that the more π-electron-donating are the substituents the larger and the more non-linear is the effect. On the other hand, lack of a similar tendency for sEDA(I) descriptor means that the σ-electron-donating or accepting character of the substituent has erratic and feeble effect on cumulative action of two substituents. This is connected to locality of the σ-electron-donating–accepting substituent effect. The rational-like concave downward decrease of the non-additivity of the sEDA(I) descriptor as the pEDA(I) descriptor is increased in the para- and ortho-homo-disubstituted benzenes denotes redistribution of the ring σ-electron density as the ring π-electron density is increased. This effect clearly shows presence of the hyperconjugation effect and reorganization of the ring σ-electron with change of the π-electron system.
Acknowledgements
Karol Hęclik is grateful for the Grant no. U-257/DS/M of the Rzeszow University of Technology for Research of Young Scientists which enabled him practices in the National Medicines Institute in 2013. The computational grant G49-12 from the Interdisciplinary Center of Mathematical and Computer Modeling (ICM) at the Warsaw University is gratefully acknowledged.Notes and references
- L. P. Hammett, J. Am. Chem. Soc., 1937, 59, 96–103 CrossRef CAS.
- L. Pauling, The Nature of the Chemical Bond, Cornell University Press, Ithaca, New York, 3rd edn, 1960 Search PubMed.
- R. S. Mulliken, J. Chem. Phys., 1934, 2, 782–793 CrossRef CAS PubMed.
- J. Hinze and H. H. Jaffe, J. Am. Chem. Soc., 1962, 84, 540–546 CrossRef CAS.
- W. Gordy, Phys. Rev., 1946, 69, 604–607 CrossRef CAS.
- A. L. Allred and E. G. Rochow, J. Inorg. Nucl. Chem., 1958, 5, 264–268 CrossRef CAS.
- R. J. Boyd and G. E. Markus, J. Chem. Phys., 1981, 75, 5385–5388 CrossRef CAS PubMed.
- F. Weinhold and C. R. Landis, Valency and Bonding: A Natural Bond Orbital Donor–Acceptor Perspective, Cambridge University Press, New York, 2005 Search PubMed.
- H. K. Hall Jr, J. Am. Chem. Soc., 1956, 78, 2570–2572 CrossRef.
- R. W. Taft, J. Chem. Phys., 1957, 26, 93–96 CrossRef CAS PubMed.
- L. Liu, Y. Fu, R. Liu, R.-Q. Li and Q.-X. Guo, J. Chem. Inf. Comput. Sci., 2004, 44, 652–657 CrossRef CAS PubMed.
- S. Mariott, W. F. Reynolds, R. W. Taft and R. D. Topsom, J. Org. Chem., 1984, 49, 959 CrossRef.
- C. G. Swain and E. C. Lupton, J. Am. Chem. Soc., 1968, 90, 4328–4337 CrossRef CAS.
- R. W. Taft and R. D. Topsom, Prog. Phys. Org. Chem., 1987, 16, 1–83 CrossRef PubMed.
- M. Charton, J. Org. Chem., 1961, 26, 735–738 CrossRef CAS.
- M. Charton, Prog. Phys. Org. Chem., 1973, 10, 81–204 CAS.
- S. J. Grabowski, T. M. Krygowski and J. Leszczyński, J. Phys. Chem. A, 2009, 113, 1105–1110 CrossRef CAS PubMed.
- S. J. Grabowski, T. M. Krygowski and M. Walczak, Chem. Phys. Lett., 2004, 400, 362–367 CrossRef CAS PubMed.
- S. J. Grabowski and T. M. Krygowski, Chem. Phys. Lett., 2004, 389, 51–57 CrossRef PubMed.
- M. Roman, J. Cz. Dobrowolski and M. Baranska, J. Chem. Inf. Model., 2011, 51, 283–295 CrossRef CAS PubMed.
- W. P. Ozimiński and J. Cz. Dobrowolski, J. Phys. Org. Chem., 2009, 22, 769–778 CrossRef PubMed.
- J. P. Foster and F. Weinhold, J. Am. Chem. Soc., 1980, 102, 7211–7218 CrossRef CAS.
- A. E. Reed, R. B. Weinstock and F. Weinhold, J. Chem. Phys., 1985, 83, 735–746 CrossRef CAS PubMed.
- A. E. Reed, L. A. Curtiss and F. Weinhold, Chem. Rev., 1988, 88, 899–926 CrossRef CAS.
- A. E. Reed and F. Weinhold, J. Chem. Phys., 1983, 78, 4066–4073 CrossRef CAS PubMed.
- E. D. Glendening, C. R. Landis and F. Weinhold, WIREs Comput. Mol. Sci., 2012, vol. 2, pp. 1–42 Search PubMed.
- R. Boyd and K. E. Edgecombe, J. Am. Chem. Soc., 1988, 110, 4182–4186 CrossRef CAS.
- R. J. Boyd and S. L. Boyd, J. Am. Chem. Soc., 1992, 114, 1652 CrossRef CAS.
- R. W. Taft, J. Am. Chem. Soc., 1957, 79, 1045–1049 CrossRef CAS.
- A. Mazurek and J. Cz. Dobrowolski, J. Org. Chem., 2012, 77, 2608–2618 CrossRef CAS PubMed.
- A. Mazurek and J. Cz. Dobrowolski, Org. Biomol. Chem., 2013, 11, 2997–3013 CAS.
- W. P. Ozimiński, T. M. Krygowski, P. W. Fowler and A. Soncini, Org. Lett., 2010, 12, 4880–4883 CrossRef PubMed.
- T. M. Krygowski, W. P. Ozimiński, M. Palusiak, P. W. Fowler and A. D. McKenzie, Phys. Chem. Chem. Phys., 2010, 12, 10740–10745 RSC.
- T. M. Krygowski, W. P. Ozimiński and M. K. Cyrański, J. Mol. Model., 2012, 18, 2453–2460 CrossRef CAS PubMed.
- T. M. Krygowski and W. P. Ozimiński, J. Mol. Model., 2011, 17, 565 CrossRef PubMed.
- W. P. Ozimiński and T. M. Krygowski, Chem. Phys. Lett., 2011, 510, 53–56 CrossRef PubMed.
- P. Cysewski, T. Jeliński, T. M. Krygowski and W. P. Oziminski, Curr. Org. Chem., 2012, 16, 1920–1933 CrossRef CAS.
- W. P. Oziminski, J. Organomet. Chem., 2012, 708–709, 10–17 CrossRef CAS PubMed.
- W. P. Ozimiński and T. M. Krygowski, Comput. Theor. Chem., 2012, 998, 46–50 CrossRef PubMed.
- T. M. Krygowski, W. P. Ozimiński and M. K. Cyrański, J. Mol. Model., 2012, 18, 2453–2460 CrossRef CAS PubMed.
- W. P. Ozimiński, T. M. Krygowski and S. Noorizadeh, Struct. Chem., 2012, 23, 931–938 CrossRef.
- W. P. Ozimiński and T. M. Krygowski, J. Phys. Org. Chem., 2013, 26, 575–582 CrossRef PubMed.
- W. P. Ozimiński, Struct. Chem., 2013, 24, 981–991 CrossRef PubMed.
- M. Jarończyk and J. Cz. Dobrowolski, Comput. Theor. Chem., 2011, 974, 9–15 CrossRef PubMed.
- T. M. Krygowski, W. P. Ozimiński and C. A. Ramsden, J. Mol. Model., 2011, 17, 1427–1433 CrossRef CAS PubMed.
- W. P. Ozimiński and T. M. Krygowski, Tetrahedron, 2011, 67, 6316–6321 CrossRef PubMed.
- W. P. Ozimiński, Comput. Theor. Chem., 2012, 980, 92–100 CrossRef PubMed.
- M. Rode and A. L. Sobolewski, J. Phys. Chem. A, 2010, 114, 11879–11889 CrossRef CAS PubMed.
- W. P. Ozimiński and T. M. Krygowski, Tetrahedron, 2011, 67, 6316–6321 CrossRef PubMed.
- T. M. Krygowski, M. A. Dobrowolski, M. K. Cyrański, W. P. Ozimiński and P. Bultinck, Comput. Theor. Chem., 2012, 984, 36–42 CrossRef CAS PubMed.
- A. Tokatli and S. Akyürekli, Struct. Chem., 2013, 24, 445–454 CrossRef CAS PubMed.
- A. N. Chermahini, B. Hosseinzadeh, A. S. Beni and A. Teimouri, Comput. Theor. Chem., 2012, 994, 97–104 CrossRef CAS PubMed.
- G. Karpińska, A. P. Mazurek and J. Cz. Dobrowolski, J. Mol. Struct.: THEOCHEM, 2011, 972, 48–56 Search PubMed.
- G. Karpińska, A. P. Mazurek and J. C. Dobrowolski, Comput. Theor. Chem., 2012, 993, 13–19 CrossRef PubMed.
- T. X. Carroll, T. D. Thomas, L. J. Saethre and K. J. Borve, J. Phys. Chem. A, 2009, 113, 3481–3490 CrossRef CAS PubMed.
- I. Hyla-Kryspin, S. Grimme, H. H. Bulker, N. M. M. Nibbering, F. Cottet and M. Schlosser, Chem.–Eur. J., 2005, 11, 1251–1256 CrossRef CAS PubMed.
- H. H. Bilker, N. M. M. Nibbering, D. Espinesa, F. Mongin and M. Schlosser, Tetrahedron Lett., 1997, 38, 8519–8522 CrossRef.
- A. Kovacs and I. Hargittai, Struct. Chem., 2000, 11, 193–201 CrossRef CAS.
- M. Shahamirian, M. K. Cyrański and T. M. Krygowski, J. Phys. Chem. A, 2011, 115, 12688–12694 CrossRef CAS PubMed.
- V. Gineityte, J. Mol. Struct.: THEOCHEM, 2001, 546, 107–117 CrossRef CAS.
- T. M. Krygowski, M. A. Dobrowolski, K. Zborowski and M. K. Cyrański, J. Phys. Org. Chem., 2006, 19, 889–895 CrossRef CAS PubMed.
- T. M. Krygowski, M. Palusiak, A. Płonka and J. E. Zachara-Horeglad, J. Phys. Org. Chem., 2007, 20, 297–306 CrossRef CAS PubMed.
- A. P. Minton, Biopolymers, 2013, 99, 239–244 CrossRef CAS PubMed.
- J. Dillen, Int. J. Quantum Chem., 2013, 18, 2143–2153 CrossRef PubMed.
- H. Fujimoto, Y. Mizutani, J. Endo and Y. A. Jinbu, J. Org. Chem., 1989, 54, 2568–2573 CrossRef CAS.
- B. Stulgies, P. Prinz, J. Magull, K. Rauch, K. Meindl, S. Rühl and A. de Meijere, Chem.–Eur. J., 2004, 11, 308–320 CrossRef CAS PubMed.
- R. Brzozowski, J. Cz. Dobrowolski, M. H. Jamróz and W. Skupiński, J. Mol. Catal. A: Chem., 2001, 170, 95–99 CrossRef CAS.
- S. Ostrowski, J. Cz. Dobrowolski, M. H. Jamróz and R. Brzozowski, Catal. Commun., 2004, 5, 733–737 CrossRef CAS PubMed.
- H. Szatyłowicz, T. M. Krygowski, C. Fonseca Guerra and F. M. Bickelhaupt, J. Comput. Chem., 2013, 34, 696–705 CrossRef PubMed.
- A. Jezierska-Mazzarello, H. Szatyłowicz and T. M. Krygowski, J. Mol. Model., 2012, 18, 127–135 CrossRef CAS PubMed.
- T. M. Krygowski, J. E. Zachara-Horeglad and M. Palusiak, J. Org. Chem., 2010, 75, 4944–4949 CrossRef CAS PubMed.
- T. M. Krygowski and J. E. Zachara-Horeglad, Tetrahedron, 2009, 65, 2010–2014 CrossRef CAS PubMed.
- E. A. Meyer, R. K. Castellano and F. Diederich, Angew. Chem., Int. Ed., 2003, 42, 1210–1250 CrossRef CAS PubMed.
- A. M. Slifkin, Charge-transfer interaction of biomolecules, Academic Press, New York, 1971 Search PubMed.
- J. E. Rode and J. Cz. Dobrowolski, Chirality, 2012, 24, 5–16 CrossRef CAS PubMed.
- P. Hobza and J. Sponer, J. Am. Chem. Soc., 2002, 124, 11802–11808 CrossRef CAS PubMed.
- J. Sponer, K. E. Riley and P. Hobza, Phys. Chem. Chem. Phys., 2008, 10, 2595–2610 RSC.
- P. Cysewski, Phys. Chem. Chem. Phys., 2008, 10, 2636–2645 RSC.
- P. Cysewski, New J. Chem., 2009, 33, 1909–1917 RSC.
- G. B. McGaughey, M. Gagné and A. K. Rappé, J. Biol. Chem., 1998, 273, 15458–15463 CrossRef CAS PubMed.
- P. Chakrabarti and R. Bhattacharyya, Prog. Biophys. Mol. Biol., 2007, 95, 83–137 CrossRef CAS PubMed.
- A. D. Becke, J. Chem. Phys., 1993, 98, 5648 CrossRef CAS PubMed.
- K. Burke, J. P. Perdew and Y. Wang, in Electronic Density Functional Theory: Recent Progress and New Directions, ed. J. F. Dobson, G. Vignale and M. P. Das, Plenum, Oxford, UK, 1998 Search PubMed.
- R. Janoschek, Pure Appl. Chem., 2001, 73, 1521–1553 CrossRef CAS.
- T. H. Dunning Jr, J. Chem. Phys., 1989, 90, 1007–1023 CrossRef PubMed.
- R. A. Kendall, T. H. Dunning and R. J. Harrison, J. Chem. Phys., 1992, 96, 6796–6806 CrossRef CAS PubMed.
- R. Ditchfield, W. J. Hehre and J. A. Pople, J. Chem. Phys., 1971, 54, 724–728 CrossRef CAS PubMed.
- R. Krishnan, J. S. Binkley, R. Seeger and J. A. Pople, J. Chem. Phys., 1980, 72, 650–654 CrossRef CAS PubMed.
- J. B. Foresman and A. E. Frisch, Exploring Chemistry with Electronic Structure Methods, Gaussian, Inc., Pittsburgh, PA, 2nd edn, 1996 Search PubMed.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery, J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, Ö. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski and D. J. Fox, GAUSSIAN 09 (Revision B.01), Gaussian, Inc., Wallingford CT, 2010 Search PubMed.
- F. A. Carey and R. J. Sundberg, Advanced Organic Chemistry – Structure and Mechanisms, Springer Science+Business Media, Berlin, 5th edn, 2007 Search PubMed.
- A. E. Reed, L. A. Curtiss and F. Weinhold, Chem. Rev., 1988, 88, 899–926 CrossRef CAS.
- P. M. Wojciechowski, Wiad. Chem., 2005, 59, 193–211 CAS.
- E. D. Glendening, A. E. Reed, J. E. Carpenter and F. Weinhold, NBO Version 3.1 Search PubMed.
- T. M. Krygowski and M. K. Cyrański, Chem. Rev., 2001, 101, 1385–1419 CrossRef CAS PubMed.
- K. Hęclik, EDA-Reader a program for calculating sEDA and pEDA descriptors from NBO Gaussian calculations, Rzeszów University of Technology, Rzeszów, 2012 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Tables giving XYZ coordinates and Gibbs free energy values for all studied molecules optimized at the DFT/B3LYP/aug-cc-pVDZ level. See DOI: 10.1039/c4ra02294g |
| This journal is © The Royal Society of Chemistry 2014 |