Tuning cadmium coordination architectures using 1,4-bis(1,2,4-triazol-1-ylmethyl)benzene and sulfoisophthalate†
Jian-Gang Ding,
Chao Yin,
Ling-Yun Zheng,
Shan-Shan Han,
Bao-Long Li* and
Bing Wu
Key Laboratory of Organic Synthesis of Jiangsu Province, College of Chemistry, Chemical Engineering and Materials Science, Soochow University, Suzhou 215123, P. R. China. E-mail: libaolong@suda.edu.cn
First published on 23rd April 2014
Abstract
In our efforts to tune Cd(II) coordination polymer architectures, three Cd(II) coordination polymers, {[Cd3(bbtz)4(sip)2(H2O)4]·12H2O}n (1), {[Cd3(bbtz)3(sip)2(H2O)6]·4H2O}n (2) and {[Cd3(bbtz)2(sip)2(H2O)8]·4H2O}n (3), were synthesized by the slow diffusion, direct reaction and hydrothermal methods using 1,4-bis(1,2,4-triazol-1-ylmethyl)benzene (bbtz) and sulfoisophthalate (sip). 1 shows a novel two-fold interpenetrating 5-connected 3D network, with a point symbol of 44·63·83. 2 displays an unusual 4-connected 2D network, with a point symbol of 42·63·8. 3 exhibits an unusual (3,3)-connected 2D network, with a point symbol of (83)2 and a 2D → 3D polythreaded network. The diverse structures demonstrate that the structures of coordination polymers can be tuned by the synthesis method. The luminescence and thermal stability were investigated.
Introduction
The rational design and synthesis of coordination polymers is of great interest in modern inorganic chemistry, owing to their potential applications as functional materials.1–3 The assembly of coordination polymer frameworks is mainly affected by the combination of a few factors, including the metal ion, the organic ligand and auxiliary ligand, the metal-to-ligand ratio, the solvent, and the reaction temperature.2–4 However, it still remains a great challenge to rationally design and construct desired coordination polymers by controlling the factors that affect their structures. The hydrothermal method is widely used for coordination polymers.5 The diffusion reaction is an effective method for the construction of coordination polymers with intriguing motifs.61,2,4-Triazole and its derivatives are very interesting ligands because they combine the coordination geometries of both pyrazole and imidazole, with regard to the arrangement of their three heteroatoms.7 Flexible bis(triazole) ligands are widely used to construct coordination polymers, because flexible ligands can adopt different conformations according to the geometric needs of the different metal ions.8 At the same time, multi-carboxylate ligands can influence the structures of the coordination polymers, owing to the fact that they can satisfy the charge-balance and even mediate the coordination of the metal centers, and more importantly, they can provide diverse ligands and versatile coordination modes.9 5-Sulfoisophthalic acid (H3sip) is a good multi-functional ligand for the construction of coordination polymers.10
In previous work, we synthesized many coordination polymers using flexible bis(triazole) ligands such as 1,2-bis(1,2,4-triazol-1-yl)ethane (bte), 1,4-bis(1,2,4-triazol-1-yl)butane (btb) and 1,4-bis(1,2,4-triazol-1-ylmethyl)benzene (bbtz) (Scheme 1).11–13 [Cd3(bbtz)6(H2O)6](BF4)6·1.75H2O shows 2D (4,4) networks and 1D ribbons of rings polycatenated in a 3D array.13a [Co(bbtz)(NO2-1,3-bdc)(H2O)]n exhibits a 3-fold interpenetrating 4-connected 65·8-CdSO4 3D network (NO2-1,3-bdc = 5-nitroisophthalate).13b With this background information, we sought to investigate the role of the synthesis method on the structures of coordination polymers. In the present work, three cadmium(II) coordination polymers {[Cd3(bbtz)4(sip)2(H2O)4]·12H2O}n (1), {[Cd3(bbtz)3(sip)2(H2O)6]·4H2O}n (2) and {[Cd3(bbtz)2(sip)2(H2O)8]·4H2O}n (3) were synthesized (bbtz = 1,4-bis(1,2,4-triazol-1-ylmethyl)benzene, H3sip = 5-sulfoisophthalic acid). 1 shows a two-fold interpenetrating 5-connected 3D network. 2 displays an unusual 4-connected 2D network. 3 exhibits an unusual (3,3)-connected 2D network, and a 2D → 3D polythreaded network.
Experimental section
Materials and physical measurements
All reagents were of analytical grade and were used without further purification. Elemental analyses for C, H and N were performed on a Perkin-Elmer 240C analyser. IR spectra were obtained for KBr pellets on a Nicolet 170SX FT-IR spectrophotometer in the 4000–400 cm−1 region. Luminescence measurements were carried out in the solid state at room temperature, and the spectra were collected with a Perkin-Elmer LS50B spectrofluorimeter. TGA was performed on a Thermal Analyst 2100 TA instrument and SDT 2960 Simultaneous TGA-DTA instrument under a N2 atmosphere at a heating rate of 10 °C min−1.Synthesis of {[Cd3(bbtz)4(sip)2(H2O)4]·12H2O}n (1)
A 5 mL aqueous solution of Cd(NO3)2·6H2O (0.2 mmol) and NaH2sip (0.2 mmol) in a test tube was adjusted to pH 6 with dilute NaOH solution. Then, a 3 mL MeOH–H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) solution and a 5 mL MeOH solution of bbtz (0.2 mmol) were carefully layered over the above solution, respectively. Colorless crystals of 1 were obtained after three weeks in a 47% yield based on the bbtz (0.049 g). Anal. calcd for C64H86Cd3N24O30S2 (1): C, 37.08; H, 4.18; N, 16.22; found: C, 37.03; H, 4.15; N, 16.20%. IR (cm−1, KBr): 3421m, 3143m, 1653m, 1604s, 1558m, 1523m, 1436m, 1280w, 1200w, 1130s, 1111w, 1015w, 998m, 772w, 729m, 675w, 622m.
1, v/v) solution and a 5 mL MeOH solution of bbtz (0.2 mmol) were carefully layered over the above solution, respectively. Colorless crystals of 1 were obtained after three weeks in a 47% yield based on the bbtz (0.049 g). Anal. calcd for C64H86Cd3N24O30S2 (1): C, 37.08; H, 4.18; N, 16.22; found: C, 37.03; H, 4.15; N, 16.20%. IR (cm−1, KBr): 3421m, 3143m, 1653m, 1604s, 1558m, 1523m, 1436m, 1280w, 1200w, 1130s, 1111w, 1015w, 998m, 772w, 729m, 675w, 622m.
Synthesis of {[Cd3(bbtz)3(sip)2(H2O)6]·4H2O}n (2)
A 5 mL aqueous solution of NaH2sip (0.2 mmol) was adjusted to pH 6 with dilute NaOH solution. Then, bbtz (0.2 mmol) in 5 mL MeOH and Cd(NO3)2]·6H2O (0.2 mmol) in 5 mL H2O were slowly added, respectively. The mixed solution was refluxed for 1 h and filtered. Colorless crystals of 2 were obtained after the filtrate was left standing for one week, in 41% yield based on bbtz (0.047 g). Anal. calc. for C52H62Cd3N18O24S2 (2): C, 36.22; H, 3.62; N, 14.62; found: C, 36.28; H, 3.60; N, 14.56%. IR (cm−1, KBr): 3421m, 3118m, 1604m, 1558m, 1519s, 1427m, 1356w, 1280w, 1213w, 1180w, 1132s, 1108w, 1038s, 1016w, 983m, 875w, 787m, 727m, 630m.Synthesis of {[Cd3(bbtz)2(sip)2(H2O)8]]·4H2O}n (3)
A solution of NaH2sip (0.2 mmol) in 5 mL H2O was adjusted to pH 6 with dilute NaOH solution. Then, bbtz (0.2 mmol) in 3 mL MeOH and Cd(NO3)2]·4H2O (0.2 mmol) in 5 mL H2O were added. The mixture was transferred to a 30 mL Teflon-lined stainless autoclave which was sealed and heated to 130 °C for 3 days, and then cooled to room temperature to give colorless crystals of 3 in 54% yield based on Cd(II) (0.055 g). Anal. calc. for C40H54Cd3N12O26S2 (3): C, 31.60; H, 3.58; N, 11.06; found: C, 31.57; H, 3.55; N, 11.04%. IR (cm−1, KBr): 3376m, 3118m, 1604s, 1558m, 1519m, 1426w, 1355s, 1280w, 1210w, 1170w, 1130s, 1108w, 1038s, 1016w, 983m, 875w, 786m, 727s, 629m.X-ray crystallography
Suitable single crystals of 1, 2 and 3 were carefully selected under an optical microscope and glued to thin glass fibers. The diffraction data were collected on a Rigaku Saturn CCD diffractometer with graphite monochromated MoKα radiation. The intensities were collected by the ω scan technique. The structures were solved by direct methods and refined with the full-matrix least-squares technique (SHELXTL-97).14 The parameters for the crystal data collection and refinement of 1, 2 and 3 are given in Table 1. Selected bond lengths and bond angles are listed in Table S1 in the ESI.†| 1 | 2 | 3 | |
|---|---|---|---|
| Formula | C64H86Cd3N24O30S2 | C52H62Cd3N18O24S2 | C40H54Cd3N12O26S2 |
| Fw | 2072.89 | 1724.52 | 1520.27 |
| T (K) | 223(2) | 223(2) | 223(2) |
| Crystal system | Triclinic | Triclinic | Triclinic |
| Space group | P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
| a (Å) | 10.187(3) | 10.3437(4) | 10.0501(16) |
| b (Å) | 13.377(4) | 13.3030(6) | 17.049(3) |
| c (Å) | 17.241(5) | 13.8717(5) | 18.433(3) |
| α (°) | 101.129(8) | 61.453(4) | 104.149(4) |
| β (°) | 100.063(7) | 83.117(3) | 93.545(3) |
| γ (°) | 93.506(5) | 88.190(3) | 90.484(4) |
| V (Å3) | 2258.7(10) | 1663.91(13) | 3055.8(9) |
| F(000) | 1054 | 868 | 1524 |
| Z | 1 | 1 | 2 |
| ρcalcd (g cm−3) | 1.524 | 1.721 | 1.652 |
| μ (mm−1) | 0.834 | 1.104 | 1.190 |
| Reflections collected | 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 974 974 |
11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 702 702 |
27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
| Unique reflections | 9035 [Rint= 0.0490] | 6827 [Rint = 0.0248] | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 823 [Rint = 0.0331] 823 [Rint = 0.0331] |
| Parameter | 523 | 478 | 834 |
| Goodness of fit | 1.094 | 1.033 | 1.066 |
| R1 [I > 2σ(I)] | 0.0782 | 0.0401 | 0.0548 |
| wR2 (all data) | 0.2190 | 0.0940 | 0.1597 |
Results and discussion
Crystal structure of {[Cd3(bbtz)4(sip)2(H2O)4]·12H2O}n (1)
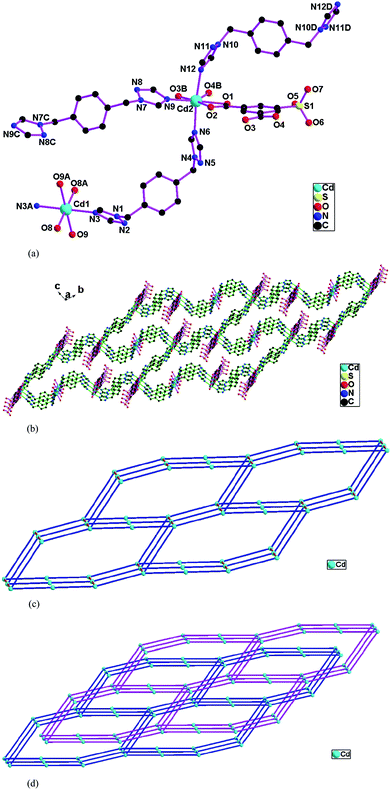
Single-crystal X-ray analysis reveals that 1 shows a novel two-fold interpenetrating 5-connected 3D network. The asymmetric unit of 1 consists of one and a half Cd(II) atoms (Cd1, Cd2), one and two halves of bbtz (N1–N6, N7–N9, N10–N12), one sip, two coordination water molecules and lattice water. Cd1 is located on an inversion center, and is six-coordinated, with four oxygen atoms from four coordination water molecules, and two triazole nitrogen atoms from two bbtz ligands, in a distorted octahedral geometry (Fig. 1a). Cd2 is coordinated by four carboxylate oxygen atoms from two sip ligands, and three triazole nitrogen atoms from three bbtz, in a distorted pentagonal bipyramid geometry.Each carboxylate group (O1O2 or O3O4) of a single sip ligand exhibits a chelating mode and coordinates one Cd2 atom (Scheme 2). Each sip ligand acts as a 2-connected node and links two Cd2 atoms with the Cd⋯Cd distance of 10.187(3). One bbtz (N1–N6) shows the gauche-conformation (Scheme 1), and joins Cd1 and Cd2 atoms with the Cd⋯Cd distance of 13.666(2). The other two bbtz ligands (N7–N9, N10–N12) exhibit the anti-conformation, and join two Cd2 atoms with the Cd⋯Cd distance of 15.737(4) and 14.062(3), respectively.
Each Cd1 atom is connected to two Cd2 atoms by two bbtz (N1–N6) bridges (Cd1 is 2-connected). Each Cd2 atom is connected to five Cd atoms (one Cd1 and four Cd2 atoms) by three bbtz (N1–N6, N7–N9, N10–N12) bridges and two sip bridges (Cd2 is 5-connected), which extend to construct a novel 5-connected 3D network (Fig. 1b). The point symbol of the 3D network is 44·63·83 (Fig. 1c).15 Because the single 3D network has large spacious voids, it allows another identical network to interpenetrate, forming a two-fold interpenetrating 3D network (Fig. 1d). Such topology is unprecedented, to our best knowledge.
Lee and coworkers synthesized two novel 5-connected coordination polymers [Ni(oba)(dia)1.5(H2O)]H2O and [Ni(Hsip)(dia)1.5(H2O)]H2O.16 [Ni(oba)(dia)1.5(H2O)]H2O consists of 2D thick layers with a 48·62 topology, and a rare (2D → 3D) polycatenated array (oba = 4,4′-oxybis(benzoate), dia = 9,10-di(1H-imidazol-1-yl)anthracene). [Ni(Hsip)(dia)1.5(H2O)]H2O exhibits a two-fold interpenetrated 3D bnn topology.
Crystal structure of {[Cd3(bbtz)3(sip)2(H2O)6]·4H2O}n (2)
2 displays an unusual 4-connected 2D network. The asymmetric unit of 2 consists of one and a half Cd(II) atoms (Cd1, Cd2), one and a half bbtz ligands (N1–N6, N7–N9), one sip, three coordination water molecules and lattice water. Cd1 is coordinated by three carboxylate oxygen atoms from two sip ligands, one coordination water oxygen atom, and two triazole nitrogen atoms from two bbtz ligands in a distorted octahedral geometry (Fig. 2a). Cd2 is located on an inversion center, and is six-coordinated with four oxygen atoms from four coordination water molecules and two triazole nitrogen atoms from two bbtz ligands, in a distorted octahedral geometry.One carboxylate group (O1O2) of each sip ligand exhibits a chelating mode (Scheme 2). The other carboxylate group (O3O4) of the sip ligand acts in a monodentate mode. Each sip ligand acts as a 2-connected node and links two Cd1 atoms with the Cd⋯Cd distance of 10.344(1). One bbtz (N1–N6) shows the gauche-conformation (Scheme 1), and joins Cd1 and Cd2 atoms with the Cd⋯Cd distance of 11.657(1). The other bbtz (N7–N9) exhibits the anti-conformation, and joins two Cd1 atoms with the Cd⋯Cd distance of 14.277(1). Each Cd2 atom is linked to two Cd1 atoms through two bbtz (N1–N6) bridges (Cd2 is 2-connected). Each Cd1 atom is connected to four Cd atoms (three Cd1 and one Cd2) by two bbtz (N1–N6, N7–N9) bridges and two sip bridges (Cd1 is 4-connected), which extend to form an unusual 4-connected 2D network (Fig. 2b). The point symbol of the 2D network is 42·63·8 (Fig. 2c).15 Such a 2D network is unusual. The 4-connected networks are usually a 2D (4,4) network,17 3D diamondoid18 or 3D 65·8-CdSO4 network.19
There are versatile hydrogen bonding interactions between the coordination water and the sip oxygen atoms, the lattice water and the sip oxygen atoms, and the coordination water and the lattice water (Table S2 in the ESI†). A 3D hydrogen bonding network is formed via these hydrogen bonding interactions (Fig. S1 in the ESI†).
Crystal structure of {[Cd3(bbtz)2(sip)2(H2O)8]·4H2O}n (3)
3 exhibits an unusual (3,3)-connected 2D network and a 2D → 3D polythreaded network. The asymmetric unit of 3 consists of three Cd(II) atoms (Cd1, Cd2, Cd3), two bbtz ligands (N1–N6, N7–N12), two sip ligands (O1–O7S1, O8–O14S2), eight coordination water molecules and lattice water. Cd1 is coordinated by four carboxylate oxygen atoms from two sip ligands, two coordination water oxygen atoms, and one bbtz triazole nitrogen atom, in a distorted pentagonal bipyramid geometry (Fig. 3a). Cd2 is six-coordinated, with three carboxylate oxygen atoms from two sip ligands, two coordination water oxygen atoms, and one bbtz triazole nitrogen atom, in a distorted octahedral geometry. Cd3 is six-coordinated, with four coordination water oxygen atoms and two bbtz triazole nitrogen atoms in a distorted octahedral geometry.The carboxylate groups (O1O2, O3O4, O10O11) of the sip ligands exhibit a chelating mode. The carboxylate group (O8O9) of the sip ligand acts in a monodentate mode (Scheme 2). All of the sip ligands act as 2-connected nodes, and link Cd1 and Cd2 atoms with the Cd⋯Cd distance of 10.191(2) and 10.268(2). One bbtz ligand (N1–N6) shows the anti-conformation (Scheme 1), and joins Cd1 and Cd3 atoms with the Cd⋯Cd distance of 14.237(2). The other bbtz ligand (N7–N12) exhibits the anti-conformation, and connects Cd2 and Cd3 atoms with the Cd⋯Cd distance of 13.230(2). The Cd3 atom is 2-connected. The bbtz and sip ligands are also 2-connected. Each Cd1 atom is linked to three Cd(II) atoms (two Cd2 and one Cd3) by two sip and one bbtz bridges (Cd1 is 3-connected). Each Cd2 atom is joined to three Cd(II) atoms (two Cd1 and one Cd3) by two sip and one bbtz bridges (Cd2 is 3-connected) which extend to form an unusual (3,3)-connected 2D network (Fig. 3b). The point symbol of the 2D network is (83)2 (Fig. 3c).15
The adjacent, highly undulated, 2D networks interweave with each other to form 2D → 3D polythreaded network (Fig. 3d). Polythreaded structures with finite components are unusual.20,21 2D → 3D polythreaded networks are unusual, and few have been reported.21 There are versatile hydrogen bonding interactions between the coordination water and the sip oxygen atoms or triazole nitrogen atoms, the lattice water and the sip oxygen atoms (Table S2 in the ESI†).
Photoluminescence properties
Due to the excellent fluorescence properties of d10 metal complexes, the solid state photoluminescence properties of 1, 2, 3, and the free bbtz ligand were investigated at room temperature, as depicted in Fig. 4. The free bbtz ligand displays an emission band at 415 nm upon excitation at 314 nm, which can probably be assigned to π–π* transitions.22 1, 2 and 3 exhibit emission bands with maxima at 369, 419 and 389 nm, respectively, upon excitation at 314 nm. Because the Cd(II) ion is difficult to oxidize or reduce, due to the d10 configuration, the emissions are neither caused by a metal-to-ligand charge transfer (MLCT) nor a ligand-to-metal charge transfer (LMCT). The emissions can be tentatively attributed to the intra-ligand charge transition.23,24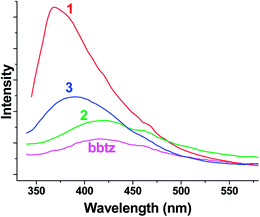 | ||
| Fig. 4 The emission spectra of 1, 2, 3 and the free bbtz ligand in the solid state at room temperature. | ||
Thermal analysis
To characterize the coordination polymers more fully in terms of thermal stability, the thermal behavior of 1, 2 and 3 were examined (Fig. S2 in the ESI†). In the TG curve of 1, the lattice and coordination water molecules were lost between 45 and 155 °C (calcd: 13.91%, found: 13.84%). The anhydrous substance was stable upon heating to 290 °C. Then, weight loss occurred continuously and did not stop until 784 °C. The residue of 1 at 850 °C should be CdO (calcd: 18.58%, found: 18.70%). 2 showed an initial weight loss of 10.28% from 40 to 150 °C, which corresponded to the loss of the lattice and coordination water molecules (calcd: 10.45%). The anhydrous substance was stable up to 295 °C. Then, weight loss occurred continuously and did not stop until 770 °C. The residue of 2 at 850 °C should be CdO (calcd: 22.34%, found: 22.52%). The lattice and coordination water molecules in 3 were lost between 45 and 150 °C (calcd: 14.22%, found: 14.06%). The anhydrous substance was stable upon heating to 303 °C. Then, weight loss occurred continuously and did not stop until 780 °C. The residue of 3 at 850 °C should be CdO (calcd: 25.34%, found: 25.31%).Conclusions
In summary, three Cd(II) coordination polymers were synthesized by the slow diffusion, direct reaction and hydrothermal methods, using the same ligands, bbtz and sip. The diverse structures demonstrate that the structures of coordination polymers can be tuned by the synthesis method.Acknowledgements
This work was supported by the National Natural Science Foundation of China (no. 21171126), the Priority Academic Program Development of Jiangsu Higher Education Institutions, the Key Laboratory of Organic Synthesis of Jiangsu Province, and the University Student Innovation Program of Soochow University.References
- (a) P. Q. Liao, D. D. Zhou, A. X. Zhu, L. Jiang, R. B. Lin, J. P. Zhang and X. M. Chen, J. Am. Chem. Soc., 2012, 134, 17380 CrossRef CAS PubMed; (b) H. C. Zhou, J. R. Long and O. M. Yaghi, Chem. Rev., 2012, 112, 673 CrossRef CAS PubMed; (c) J. Zhang and C. Y. Su, Coord. Chem. Rev., 2013, 257, 1373 CrossRef CAS PubMed; (d) M. Du, C. P. Li, C. S. Liu and S. M. Fang, Coord. Chem. Rev., 2013, 257, 1282 CrossRef CAS PubMed; (e) Y. J. Cui, Y. F. Yue, G. D. Qian and B. L. Chen, Chem. Rev., 2012, 112, 1126 CrossRef CAS PubMed.
- (a) Z. G. Guo and R. Cao, J. Am. Chem. Soc., 2009, 131, 6894 CrossRef CAS PubMed; (b) H. Wu, H. Y. Liu, Y. Y. Liu, J. Yang, B. Liu and J. F. Ma, Chem. Commun., 2011, 47, 1818 RSC; (c) K. Otsubo, T. Haraguchi, O. Sakata, A. Fujiwara and H. Kitagawa, J. Am. Chem. Soc., 2012, 134, 9605 CrossRef CAS PubMed; (d) Y. Bu, F. L. Jiang, K. Zhou, X. J. Li, L. J. Zhang, Y. L. Gai and M. C. Hong, CrystEngComm, 2013, 15, 8426 RSC; (e) H. Xu, W. W. Bao, Y. Xu, X. L. Liu, X. Shen and D. R. Zhu, CrystEngComm, 2012, 14, 5720 RSC; (f) H. B. Zhou, J. H. Yao, X. P. shen, H. Zhou and A. H. Yuan, RSC Adv., 2014, 4, 61 RSC; (g) Y. X. Tan, Y. P. He, H. Wang and J. Zhang, RSC Adv., 2014, 4, 1480 RSC.
- (a) A. Laguna, T. Lasanta, J. M. Lopezde-Luzuriaga, M. Monge, P. Naumov and M. E. Olmos, J. Am. Chem. Soc., 2010, 132, 456 CrossRef CAS PubMed; (b) Z. Z. Lu, R. Zhang, Y. Z. Li, Z. J. Guo and H. G. Zheng, J. Am. Chem. Soc., 2011, 133, 4172 CrossRef CAS PubMed; (c) L. L. Wen, J. B. Zhao, K. L. Lv, Y. H. Wu, K. J. Deng, X. K. Leng and D. F. Li, Cryst. Growth Des., 2012, 12, 1603 CrossRef CAS; (d) P. Yang, X. He, M. X. Li, Q. Ye, J. Z. Ge, Z. X. Wang, S. R. Zhu, M. Shao and H. L. Cai, J. Mater. Chem., 2012, 22, 2398 RSC; (e) F. Y. Yi, S. Dang, W. T. Yang and Z. M. Sun, CrystEngComm, 2013, 15, 8320 RSC.
- (a) L. F. Ma, L. Y. Wang, D. H. Lu and S. R. Batten, Cryst. Growth Des., 2009, 9, 1741 CrossRef CAS; (b) F. Luo, M. B. Luo and Y. H. Liu, CrystEngComm, 2010, 12, 1750 RSC; (c) S. L. Li, K. Tan, Y. Q. Lan, J. S. Qin, M. N. Li, D. Y. Du, H. Y. Zang and Z. M. Su, Cryst. Growth Des., 2010, 10, 1699 CrossRef CAS; (d) M. Chen, Y. Lu, J. Fan, G. C. Lv, Y. Zhao and W. Y. Sun, CrystEngComm, 2012, 14, 2015 RSC; (e) Y. Zhang, J. Yang, Y. Yang, J. Guo and J. F. Ma, Cryst. Growth Des., 2012, 12, 4060 CrossRef CAS.
- (a) T. Yamada, S. Iwakiri, T. Hara, K. Kanaizuka, M. Kurmoo and H. Kitagawa, Cryst. Growth Des., 2011, 11, 1798 CrossRef CAS; (b) L. Chen, J. Y. Guo, X. Xu, W. W. Ju, D. Zhang, D. R. Zhu and Y. Xu, Chem. Commun., 2013, 49, 9728 RSC; (c) X. He, X. P. Lu, Y. Y. Tian, M. X. Li, S. R. Zhu, F. F. Xing and R. E. Morris, CrystEngComm, 2013, 15, 9437 RSC; (d) S. Zhang, N. X. Sun, L. Li, Z. B. Han and Y. Z. Zheng, RSC Adv., 2014, 4, 5740 RSC; (e) K. K. Bisht, Y. Rachuri, B. Parmar and E. Suresh, RSC Adv., 2014, 4, 7352 RSC.
- (a) X. Zhu, X. G. Liu, B. L. Li and Y. Zhang, CrystEngComm, 2009, 11, 997 RSC; (b) Y. Ling, L. Zhang, J. Li and M. Du, CrystEngComm, 2011, 13, 768 RSC; (c) S. Y. Moon, T. H. Noh and O. S. Jung, CrystEngComm, 2013, 15, 3854 RSC.
- G. Aromí, L. A. Barrios, O. Roubeau and P. Gamez, Coord. Chem. Rev., 2011, 255, 485 CrossRef PubMed.
- (a) Y. Garcia, C. Bravic, C. Gieck, D. Chasseau, W. Tremel and P. Gutlich, Inorg. Chem., 2005, 44, 9723 CrossRef CAS PubMed; (b) J. P. Zhang and X. M. Chen, Chem. Commun., 2006, 1689 RSC; (c) X. Zhu, Y. F. Feng, M. Li, B. L. Li and Y. Zhang, CrystEngComm, 2012, 14, 79 RSC; (d) M. L. Han, J. G. Wang, L. F. Ma, H. Guo and L. Y. Wang, CrystEngComm, 2012, 14, 2691 RSC; (e) N. Wang, J. G. Ma, W. Shi and P. Cheng, CrystEngComm, 2012, 14, 5198 RSC; (f) J. Zhao, D. S. Li, X. J. Ke, B. Liu, K. Zou and H. M. Hu, Dalton Trans., 2012, 41, 2560 RSC; (g) X. R. Meng, Y. L. Song, H. W. Hou, H. Y. Han, B. Xiao, Y. T. Fan and Y. Zhu, Inorg. Chem., 2004, 43, 3528 CrossRef CAS PubMed.
- (a) X. Y. Lu, J. W. Ye, W. Li, W. T. Gong, L. J. Yang, Y. Lin and G. L. Ning, CrystEngComm, 2012, 14, 1337 RSC; (b) K. L. Zhang, C. T. Hou, J. J. Song, Y. Deng, L. Li, S. W. Ng and G. W. Diao, CrystEngComm, 2012, 14, 590 RSC; (c) S. Sengupta, S. Ganguly, A. Goswami, P. K. Sukul and R. Mondal, CrystEngComm, 2013, 15, 8353 RSC; (d) X. L. Wang, L. L. Hou, J. W. Zhang, J. X. Zhang, G. C. Liu and S. Yang, CrystEngComm, 2012, 14, 3936 RSC; (e) L. Zhou, Y. S. Xue, Y. Xu, J. Zhang and H. B. Du, CrystEngComm, 2013, 15, 7315 RSC; (f) H. Y. Ge, Y. F. Peng, J. G. Ding, B. L. Li and Y. Zhang, Chin. J. Chem., 2012, 30, 1479 CrossRef CAS.
- (a) A. D. Kulynych and G. K. H. Shimizu, CrystEngComm, 2002, 4, 102 RSC; (b) Q. Y. Liu, D. Q. Yuan and L. Xu, Cryst. Growth Des., 2007, 7, 1832 CrossRef CAS; (c) H. X. Guo, X. Z. Li, S. K. Huang, Y. Ling and W. Weng, Inorg. Chem. Commun., 2010, 13, 262 CrossRef CAS PubMed; (d) M. Li, S. Zhao, Y. F. Peng, B. L. Li and H. Y. Li, Dalton Trans., 2013, 42, 9771 RSC; (e) J. G. Ding, X. Zhu, Y. F. Cui, N. Liang, P. P. Sun, Q. Chen, B. L. Li and H. Y. Li, CrystEngComm, 2014, 16, 1632 RSC.
- (a) X. Zhu, S. Zhao, Y. F. Peng, B. L. Li and B. Wu, CrystEngComm, 2013, 15, 9154 RSC; (b) X. Zhu, Q. Chen, Z. Yang, B. L. Li and H. Y. Li, CrystEngComm, 2013, 15, 471 RSC; (c) X. Y. Wang, B. L. Li, X. Zhu and S. Gao, Eur. J. Inorg. Chem., 2005, 3277 CrossRef CAS.
- (a) L. Y. Wang, Y. Yang, K. Liu, B. L. Li and Y. Zhang, Cryst. Growth Des., 2008, 8, 3902 CrossRef CAS; (b) X. G. Liu, L. Y. Wang, X. Zhu, B. L. Li and Y. Zhang, Cryst. Growth Des., 2009, 9, 3997 CrossRef CAS; (c) X. Zhu, L. Y. Wang, X. G. Liu, J. Wang, B. L. Li and H. Y. Li, CrystEngComm, 2011, 13, 6090 RSC.
- (a) B. L. Li, Y. F. Peng, B. Z. Li and Y. Zhang, Chem. Commun., 2005, 2333 RSC; (b) X. Zhu, P. P. Sun, J. G. Ding, B. L. Li and H. Y. Li, Cryst. Growth Des., 2012, 12, 3992 CrossRef CAS; (c) Y. F. Peng, H. Y. Ge, B. Z. Li, B. L. Li and Y. Zhang, Cryst. Growth Des., 2006, 6, 994 CrossRef CAS.
- G. M. Sheldrick, Acta Crystallogr., Sect. A: Found. Crystallogr., 2008, 64, 112 CrossRef CAS PubMed.
- (a) V. A. Blatov, M. O'Keeffe and D. M. Proserpio, CrystEngComm, 2010, 12, 44 RSC; (b) E. V. Alexandrov, V. A. Blatov, A. V. Kochetkova and D. M. Proserpio, CrystEngComm, 2011, 13, 3947 RSC; (c) M. O'Keeffe and O. M. Yaghi, Chem. Rev., 2012, 112, 675 CrossRef PubMed.
- C. H. Ke and H. M. Lee, CrystEngComm, 2012, 14, 4157 RSC.
- P. P. Sun, S. Zhao, Z. Yang, B. L. Li and B. Wu, Chin. J. Chem., 2012, 30, 1813 CrossRef CAS.
- (a) S. R. Batten, CrystEngComm, 2001, 3, 67 RSC; (b) X. J. Li, R. Cao, D. F. Sun, W. H. Bi, Y. Q. Wang, X. Li and M. C. Hong, Cryst. Growth Des., 2004, 4, 775 CrossRef CAS; (c) J. G. Ding, X. G. Liu, B. L. Li, L. Y. Wang and Y. Zhang, Inorg. Chem. Commun., 2008, 11, 1079 CrossRef CAS PubMed.
- (a) N. L. Rosi, J. Kim, M. Eddaoudi, B. L. Chen, M. O'Keeffe and O. M. Yaghi, J. Am. Chem. Soc., 2005, 127, 1504 CrossRef CAS PubMed; (b) K. M. Blake, L. L. Johnston, M. A. Braverman, J. H. Nettleman, L. K. Sposato and R. L. LaDuca, Inorg. Chim. Acta, 2010, 363, 2233 CrossRef CAS PubMed; (c) J. N. Rebilly, P. W. Gardner, G. R. Darling, J. Bacsa and M. J. Rosseinsky, Inorg. Chem., 2008, 47, 9390 CrossRef CAS PubMed; (d) H. P. Wang, X. G. Liu, X. Zhu, B. L. Li and B. Wu, J. Coord. Chem., 2011, 64, 4254 CrossRef CAS.
- (a) S. R. Batten and R. Robson, Angew. Chem., Int. Ed., 1998, 37, 1460 CrossRef; (b) L. Carlucci, G. Ciani and D. M. Proserpio, Coord. Chem. Rev., 2003, 246, 247 CrossRef CAS.
- (a) X. Wang, C. Qin, E. Wang, Y. Li, Z. Su, L. Xu and L. Carlucci, Angew. Chem., Int. Ed., 2005, 44, 5824 CrossRef CAS PubMed; (b) L. P. Zhang, J. F. Ma, J. Yang, Y. Y. Pang and J. C. Ma, Inorg. Chem., 2010, 49, 1535 CrossRef CAS PubMed; (c) H. D. Guo, D. F. Qiu, X. M. Guo, G. L. Zheng, X. Wang, S. Dang and H. J. Zhang, CrystEngComm, 2009, 11, 2425 RSC; (d) Y. Y. Liu, Z. H. Wang, J. Yang, B. Liu, Y. Y. Liu and J. F. Ma, CrystEngComm, 2011, 13, 3811 RSC; (e) J. H. Qin, L. F. Ma, Y. Hu and L. Y. Wang, CrystEngComm, 2012, 14, 2891 RSC; (f) Y. F. Cui, P. P. Sun, Q. Chen, B. L. Li and H. Y. Li, CrystEngComm, 2012, 14, 4161 RSC.
- (a) F. Wang, Z. S. Liu, H. Yang, Y. X. Tan and J. Zhang, Angew. Chem., Int. Ed., 2011, 50, 450 CrossRef CAS PubMed; (b) H. X. Yang, T. F. Liu, M. N. Cao, H. F. Li, S. Y. Gao and R. Cao, Chem. Commun., 2010, 46, 2419 Search PubMed; (c) T. Wen, D. X. Zhang and J. Zhang, Inorg. Chem., 2013, 52, 12 CrossRef CAS PubMed.
- (a) P. Du, Y. Yang, J. Yang, Y. Y. Liu, W. Q. Kan and J. F. Ma, CrystEngComm, 2013, 15, 6986 RSC; (b) H. Wu, B. Liu, J. Yang, H. Y. Liu and J. F. Ma, CrystEngComm, 2011, 13, 3661 RSC.
- (a) S. Sengupta, S. Ganguly, A. Goswami, P. K. Sukul and R. Mondal, CrystEngComm, 2013, 15, 8353 RSC; (b) A. J. Calahorro, A. Salinas-Castillo, J. M. Seco, J. Zuniga, E. Colacio and A. Rodriguez-Dieguez, CrystEngComm, 2013, 15, 7336 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Selected bond lengths and angles, hydrogen bond, additional figures for the crystal structures and TG curve. CCDC 991340–991342. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c4ra02291b |
| This journal is © The Royal Society of Chemistry 2014 |