The tribological behaviour and tribochemical study of B–N type borate esters in rapeseed oil—compound versus salt†
Abstract
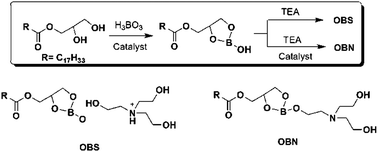
Two novel borate ester additives, (2-(2-(bis(2-hydroxyethyl)amino)ethoxy)-1,3,2-dioxaborolan-4-yl)methyl oleate and a tris(2-hydroxyethyl)amine salt of (2-hydroxy-1,3,2-dioxaborolan-4-yl)methyl oleate were prepared and used as anti-wear and extreme pressure agents in rapeseed oil. The tribological performance was evaluated using a four-ball machine. The results show that the additives possess high anti-wear and extreme pressure properties. XANES, XPS and AFM were used to analyse the composition and structure of boundary films at the worn surfaces. The results for the compound and salt of borate esters are compared, and it is shown that the boundary films formed by compound or salt are similar and mainly composed of B2O3.
- This article is part of the themed collection: Tribology

 Please wait while we load your content...
Please wait while we load your content...