Highly efficient inverted polymer solar cells using fullerene derivative modified TiO2 nanorods as the buffer layer†
Abstract
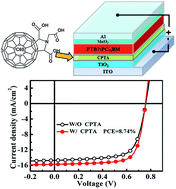
We reported a significant improvement in the efficiency of polymer solar cells by introducing C60 pyrrolidine tris-acid (CPTA) to modify the interface between inorganic TiO2 nanorods and the organic active layer. The devices with CPTA-modified TiO2 as the cathode buffer layer exhibited a power conversion efficiency (PCE) as high as 8.74%, accounting for a 12.8% enhancement compared with the bare TiO2 based devices (7.75%) in the polymer thieno[3,4-b] thiophene/benzodithiophene:[6,6]-phenyl C71-butyric acid methyl ester (PTB7:PC71BM) system. We found that the CPTA layer improves the surface properties of the bare TiO2 film so that charge transfer between the active layer and the TiO2 layer is enhanced.

 Please wait while we load your content...
Please wait while we load your content...