DOI:
10.1039/C4RA02227K
(Paper)
RSC Adv., 2014,
4, 22946-22950
Cholinium ionic liquids as cheap and reusable catalysts for the synthesis of coumarins via Pechmann reaction under solvent-free conditions†
Received
14th March 2014
, Accepted 13th May 2014
First published on 13th May 2014
Abstract
A cheap cholinium ionic liquid N,N′-dimethylaminoethanol hydrosulfate ([N112OH][HSO4]) was found to be an efficient and reusable catalyst for the Pechmann condensation under solvent-free conditions. The coumarin products can be simply separated and the ionic liquid catalyst can be recycled and reused for at least six runs without noticeable decrease in the catalytic activity. The UV-vis studies suggested that the increase of the acidity and the introduction of a hydroxyl group to the cation benefit the existence of the keto tautomer of ethyl acetoacetate, which then leads to the excellent performance of [N112OH][HSO4].
1. Introduction
Coumarin is the core unit of a number of natural products and exhibits various biological activities such as antimicrobial,1 antithrombotic,2 anticancer,3 anti-HIV,4 antioxidant5 and central nervous system modulating activities.6 It has been widely used in pharmaceuticals, perfumes, agrochemicals, and cosmetics. The synthesis of coumarin derivatives has received great attention in the synthetic organic and medicinal chemistry, and several routes including Pechmann,7 Perkin,8 Knoevenagel,9 Reformatsky,10 and Witting reactions11 have been developed. Among these routes, Pechmann reaction is the most widely used method since it proceeds from simple and available starting materials of phenols and β-ketoesters. However, acidic catalysts have to be used such as concentrated H2SO4,12 P2O5,13 trifluoroacetic acid,14 TiCl4,15 HClO4/SiO2,16 phosphotungstic acid,17 Nafion resin,18 Al-MCM-41,19 superacidic sulfated zirconia,20 superacid-functionalized mesoporous nanocages,21 nanostructure sulfated tin oxide,22 zirconium phosphotungstate,23 SnCl2,24 and sulfonic acid-functionalized ionic liquid.25,26 Most of these catalytic systems suffered from severe drawbacks such as the use of a large amount of catalyst, frequently long reaction time and low yields, sometimes high-cost for the preparation of catalyst and the difficulty or even impossibility in the catalyst reutilization. Therefore, it is still in great necessary to develop low-cost and highly efficient catalytic systems for the synthesis of coumarins with the possibility to reutilize the catalyst.
Due to the high biocompatibilility, wide resource and low preparation cost, cholinium-based ionic liquids (CILs) have been widely used in electrochemistry,27 metal processing,28 ionothermal synthesis,29 and catalytic procedures.30–33 As a continuous work on the utilization of CILs in green catalysis,30,32,33 the catalytic performance of a series of CILs on the synthesis of coumarins through the Pechmann reaction were investigated in this work. It was found that the existence of hydroxyl group on the cation and the enhancement in the acidity strength of the anions favored the catalytic activity for the Pechmann reaction, and the ionic liquid with acidic anion of hydrogen sulfate and protic cation of N,N-dimethylaminoethanol ([N112OH][HSO4]) was found to be an efficient and reusable catalyst for the synthesis of coumarins under solvent-free conditions. This catalytic system is cheap and affordable with the advantages of easy experimental and work-up procedures and no use of toxic catalyst and solvents, and the catalyst can be recovered and reused at least six runs without significant activity decrease.
2. Experimental section
2.1 Materials and methods
All chemicals of AR grade were commercially available and used without further purification. The synthesis methods for the ionic liquids were similar to the procedures reported in our previous work.30,33 According to the procedure described in literature,34 the deep eutectic mixtures of choline chloride and diacids were prepared at the molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 through simple mixing at 110 °C under stirring for 2 hours. Melting points were determined on a XRC-1 Microscopic Melting Point Measurer without correction. 1H NMR spectra were recorded on a Bruker AV-400 instrument at room temperature by using TMS as internal standard. For easy understanding, chemical structures for the cation of cholinium-based ionic liquids were shown in Scheme 1.
1 through simple mixing at 110 °C under stirring for 2 hours. Melting points were determined on a XRC-1 Microscopic Melting Point Measurer without correction. 1H NMR spectra were recorded on a Bruker AV-400 instrument at room temperature by using TMS as internal standard. For easy understanding, chemical structures for the cation of cholinium-based ionic liquids were shown in Scheme 1.
 |
| | Scheme 1 The cation structure of cholinium-based ionic liquids. | |
2.2 Typical procedures for the Pechmann reaction catalyzed by CILs
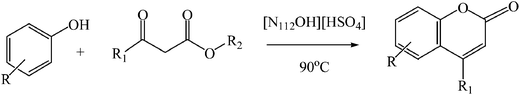
A mixture of phenol (2 mmol), ethyl acetoacetate (2.2 mmol) and ionic liquid (0.1 mmol) was added into a 10 mL round-bottom flask and stirred at 90 °C for the desired reaction time. After the reaction was completed as monitored by TLC, the reaction mixture was cooled to room temperature, and ice-cold water was added to the mixture to precipitate the product. The crude product was then recrystallized from ethanol to give pure product. The ionic liquids contained in the filtrate can be reused for the next run after the evaporation of water.
2.3 UV-vis experiments
The UV-vis spectra were recorded using a TU-1900 UV-vis spectrometer to detect the keto–enol tautomeric equilibrium of ethyl acetoacetate (EAA). The concentration of EAA in methanol was kept at 5 × 10−4 mol L−1, and the molar ratio of EAA to ionic liquid was kept at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.05. The maximum absorbance of the enol tautomer of EAA is at 244 nm.
0.05. The maximum absorbance of the enol tautomer of EAA is at 244 nm.
3. Results and discussion
3.1 The catalytic activity of different ionic liquids on the Pechmann reaction
The reaction between resorcinol and EAA was selected as a model system to study the catalytic activity of different ionic liquids on the Pechmann reaction, and the results are collected in Table 1. It was found that the ionic liquids with cholinium cation and weak acidic anions have no significant catalytic activity on the Pechmann reaction (entries 1–3, Table 1), while the ionic liquids with strong acidic anion of [HSO4]− showed moderate catalytic activity and 50% isolated yield could be obtained for the target compound in 12 hours (entry 4, Table 1). This suggests that acidity of the ionic liquids is essential for the Pechmann reaction. Then the catalytic activities of several acidic deep eutectic mixtures based on choline chloride on the Pechmann reaction were also studied (entries 5–7, Table 1). Compared with [choline][HSO4], [N112OH][HSO4] has stronger acidity,33 thus showing excellent catalytic activity for the Pechmann reaction, and 87% isolated yield has been observed for the target compound within 1.5 hours (entry 8, Table 1). However, the ionic liquids [N112OH][H2PO4] and [N112OH]Cl showed no catalytic activity (entries 9 and 10, Table 1) although they have the same cation with [N112OH][HSO4]. This is because their acidity is weaker than the ionic liquid composed with HSO4 anion as suggested by their Hammett acidity functions.33 On the other hand, the ionic liquid composed of [HSO4] anion and N,N-dimethylethylamine cation ([N112][HSO4]) can only get 50% isolated yield after 4 hours of reaction (entry 11, Table 1). These results suggest that besides the acidity of the anion, hydroxyl group on the cation of the ionic liquid is also important for the catalysis of this reaction.
Table 1 The catalytic activity of different ionic liquids on the Pechmann reaction
3.2 The influences of the catalyst amount and reaction temperature on the catalytic activity of ionic liquids
The influence of the catalyst amount on the catalytic activity of [N112OH][HSO4] on the Pechmann reaction was studied and the results were given in Table 2. It was interesting to find that the catalytic activity of the ionic liquid increased with the decrease of the molar ratio of ionic liquid to resorcinol from 1.5 to 0.05 (entries 1–5, Table 2), while the further decrease in the amount of ionic liquid from 0.05 to 0.02 led to the decrease of the catalytic activity (entry 6, Table 2). This suggests that the optimal molar ratio of ionic liquid to resorcinol should be at 0.05. Then the influence of the temperature on the reaction was studied by using the optimized catalyst amount. It was shown that the decrease in temperature reduced the catalytic activity (entries 6–8, Table 2). However, when the temperature was increased to 120 °C, the enhancement in catalytic activity accompanied by the decrease in selectivity (detected by TLC), and only 90% isolated yield was found for the target compound (entry 9, Table 2).
Table 2 The influence of catalyst amount and temperature on the model reaction
| Entry |
Molar ratio (ionic liquid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) resorcinol) resorcinol) |
Reaction temperature (°C) |
Reaction time (h) |
Isolated yield (%) |
| 1 |
1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
90 |
2 |
86 |
| 2 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
90 |
2 |
87 |
| 3 |
0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
90 |
3.5 |
94 |
| 4 |
0.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
90 |
3.5 |
98 |
| 5 |
0.05![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
90 |
3 |
99 |
| 6 |
0.02![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
90 |
3.5 |
90 |
| 7 |
0.05![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
60 |
5.5 |
75 |
| 8 |
0.05![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
25 |
5.5 |
— |
| 9 |
0.05![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
120 |
2 |
90 |
3.3 The effect of ionic liquid on the keto–enol tautomeric equilibria of EAA and the plausible reaction mechanism for the Pechmann reaction
According to the well accepted reaction mechanism for the acid catalyzed Pechmann reaction, the increased polarization of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond of β-ketoesters would benefit to its reactivity. When it comes to EAA, the polarization change of C
O bond of β-ketoesters would benefit to its reactivity. When it comes to EAA, the polarization change of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O will affect its keto–enol tautomeric equilibrium. Therefore, we studied the effect of several ionic liquids with [HSO4] anion on the keto–enol tautomeric equilibrium of EAA in methanol and the results are illustrated in Fig. 1. It can be seen that when the selected ionic liquids were added to the methanol solution of EAA, absorbance of the enol decreased in the sequence of [choline][HSO4] > [N112][HSO4] > [N112OH][HSO4]. This suggests that the addition of ionic liquid will increase the keto content in the tautomeric equilibrium and ionic liquid [N112OH][HSO4], which has stronger acidity than [choline][HSO4] and a hydroxyl group on the cation, is the most favorable for the existence of keto. Therefore, a plausible reaction mechanism was proposed and illustrated in Fig. 2. It is suggested that the hydroxyl group on the cation of [N112OH][HSO4] acts as a hydrogen bond donor to polarize the carbonyl group of EAA and increase keto content of EAA. This makes the carbonyl group more susceptible to the subsequent nucleophilic attacks by resorcinol.
O will affect its keto–enol tautomeric equilibrium. Therefore, we studied the effect of several ionic liquids with [HSO4] anion on the keto–enol tautomeric equilibrium of EAA in methanol and the results are illustrated in Fig. 1. It can be seen that when the selected ionic liquids were added to the methanol solution of EAA, absorbance of the enol decreased in the sequence of [choline][HSO4] > [N112][HSO4] > [N112OH][HSO4]. This suggests that the addition of ionic liquid will increase the keto content in the tautomeric equilibrium and ionic liquid [N112OH][HSO4], which has stronger acidity than [choline][HSO4] and a hydroxyl group on the cation, is the most favorable for the existence of keto. Therefore, a plausible reaction mechanism was proposed and illustrated in Fig. 2. It is suggested that the hydroxyl group on the cation of [N112OH][HSO4] acts as a hydrogen bond donor to polarize the carbonyl group of EAA and increase keto content of EAA. This makes the carbonyl group more susceptible to the subsequent nucleophilic attacks by resorcinol.
 |
| | Fig. 1 The influence of ionic liquids on the keto–enol tautomeric equilibria of EAA. | |
 |
| | Fig. 2 The plausible reaction mechanism. | |
3.4 The synthesis of coumarins via Pechmann reaction catalyzed by [N112OH][HSO4]
The reactivity of other phenols with β-ketoesters using [N112OH][HSO4] as catalyst was also investigated and the results were collected in Table 3. It can be seen that dihydric phenol and trihydric phenol can react with EAA smoothly, giving target compound almost quantitative yields (entries 1, 6–7, Table 3), and their corresponding reactions with methylacetoacetate (MAA) can also arrive at excellent isolated yield (entries 8–10, Table 3). However, the introduce of weak electro-donating group such as methyl group led to the decrease of catalytic activity (entries 4–5, Table 3), and the reactivity of monohydric phenol with electro-withdrawing groups such as –NH2 and CH3O– are also lowered compared with dihydric phenol and trihydric phenol (entries 2–3, Table 3). The reaction between resorcinol and other keto ester such as ethyl 2-methylacetoacetate (EMAA) can also give moderate yield under the same catalytic conditions (entry 12), but its reaction with methyl 4-methoxyacetoacetate (MMOAA) only proceed with low yield.
Table 3 The synthesis of coumarins derivatives
3.5 The reusability of [N112OH][HSO4] catalyst in Pechmann reaction
After the reaction was completed, the recovered ionic liquid catalyst [N112OH][HSO4] from the filtrate was concentrated by rotary evaporation, dried under vacuum at 50 °C and then utilized for the next run of Pechmann reaction between resorcinol and EAA. It was found from Fig. 3 that there was no significant activity decrease after six runs for the model reaction.
 |
| | Fig. 3 The reutilization of [N112OH][HSO4]. | |
4. Conclusions
In summary, the catalytic activities of a series of cholinium-based ionic liquids on the Pechmann reaction were investigated, and a cheap and reusable ionic liquid, [N112OH][HSO4], was found to be an highly efficient catalyst for the Pechmann condensation reaction under solvent-free condition. The excellent catalytic performance of [N112OH][HSO4] on the Pechmann reaction was suggested to come from the hydrogen bond activation effect of the cation and the acidity of the anion. This catalytic methodology benefits from several green aspects such as no use of toxic catalysts and solvents, ease of preparation, recovery and reutilization of the catalyst, simple experimental procedure and high chemoselectivity. Further work about the utilization of this class of ionic liquids to catalyze other organic transformations is in progress in our laboratory.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (21373079, 21003039).
References
- S. V. Laxmi, B. S. Kuarm and B. Rajitha, Med. Chem. Res., 2013, 22, 768 CrossRef.
- A. K. Mitra, A. De, N. Karchaudhuri, S. K. Misra and A. K. Mukhopadhyay, J. Indian Chem. Soc., 1998, 75, 666 CAS.
- C. J. Wang, Y. J. Hsieh, C. Y. Chu, Y. L. Lin and T. H. Tseng, Cancer Lett., 2002, 183, 163 CrossRef CAS.
- I. Kostova, Curr. HIV Res., 2006, 4, 347 CrossRef CAS.
- C. Xiao, X. Y. Luo, D. J. Li, H. Lu, Z. Q. Liu, Z. G. Song and Y. H. Jin, Eur. J. Med. Chem., 2012, 53, 159 CrossRef CAS PubMed.
- M. Noeldner, H. Hauer and S. S. Chatterjee, Drugs Future, 1996, 21, 779 CAS.
- H. von Pechmann and C. Duisberg, Chem. Ber., 1984, 17, 929 CrossRef.
- J. R. Johnson, Org. React., 1942, 1, 210 Search PubMed.
- G. Jones, Org. React., 1967, 15, 204 CAS.
- R. L. Shriner, Org. React., 1942, 1, 1 Search PubMed.
- I. Yavari, R. Hekmat-Shoar and A. Zonouzi, Tetrahedron Lett., 1998, 39, 2391 CrossRef CAS.
- E. C. Horning, Organic Synthesis, Wiley, New York, 1955, vol. 3, p. 281 Search PubMed.
- F. W. Canter, F. H. Curd and A. Robertson, J. Chem. Soc., 1931, 1245 RSC.
- L. L. Woods and J. Sapp, J. Org. Chem., 1962, 27, 3703 CrossRef CAS.
- H. Valizadeha and A. Shockravi, Tetrahedron Lett., 2005, 46, 3501 CrossRef PubMed.
- M. Maheswara, V. Siddaiah, G. L. V. Damu, Y. K. Rao and C. V. Rao, J. Mol. Catal. A: Chem., 2006, 255, 49 CrossRef CAS PubMed.
- R. S. Keri, K. M. Hosamani and H. R. S. Reddy, Catal. Lett., 2009, 131, 321 CrossRef CAS.
- R. Hinze, M. C. Laufer, W. F. Hoelderich, W. Bonrath and T. Netscher, Catal. Today, 2009, 140, 105 CrossRef CAS PubMed.
- S. Sudha, K. Venkatachalam, S. Vishnu Priya, J. Herbert Mabel, M. Palanichamy and V. Murugesan, J. Mol. Catal. A: Chem., 2008, 291, 22 CrossRef CAS PubMed.
- G. D. Yadav, N. P. Ajgaonkar and A. Varma, J. Catal., 2012, 292, 99 CrossRef CAS PubMed.
- P. Kalita, B. Sathyaseelan, A. Mano, S. M. Javaid Zaidi, M. A. Chari and A. Vinu, Chem.–Eur. J., 2010, 16, 2843 CrossRef CAS PubMed.
- A. I. Ahmed, S. A. El-Hakam, A. S. Khder and W. S. A. El-Yazeed, J. Mol. Catal. A: Chem., 2013, 366, 99 CrossRef CAS PubMed.
- S. Ghodke and U. Chudasama, Appl. Catal., A, 2013, 453, 219 CrossRef CAS PubMed.
- K. K. Upadhyay, R. K. Mishra and A. Kumar, Catal. Lett., 2008, 121, 118 CrossRef CAS PubMed.
- S. Das, A. Majee and Al. Hajra, Green Chem. Lett. Rev., 2011, 4, 349 CrossRef CAS.
- N. G. Khaligh, Catal. Sci. Technol., 2012, 2, 1633 CAS.
- C. A. Nkuku and R. J. LeSuer, J. Phys. Chem. B, 2007, 111, 13271 CrossRef CAS PubMed.
- A. P. Abbott, G. Frisch, J. Hartley and K. S. Ryder, Green Chem., 2011, 13, 471 RSC.
- E. R. Cooper, C. D. Andrews, P. S. Wheatley, P. B. Webb, P. Wormald and R. E. Morris, Nature, 2004, 430, 1012 CrossRef CAS PubMed.
- A. L. Zhu, R. X. Liu, L. J. Li, L. Y. Li, L. Wang and J. J. Wang, Catal. Today, 2013, 200, 17 CrossRef CAS PubMed.
- A. P. Abbott, T. J. Bell, S. Handa and B. Stoddart, Green Chem., 2005, 7, 705 RSC.
- A. L. Zhu, L. J. Li, J. J. Wang and K. L. Zhuo, Green Chem., 2011, 13, 1244 RSC.
- A. L. Zhu, Q. Q. Li, L. J. Li and J. J. Wang, Catal. Lett., 2013, 143, 463 CrossRef CAS.
- A. P. Aboott, D. Boothby, G. Capper, D. L. Davies and R. K. Rasheed, J. Am. Chem. Soc., 2004, 126, 9142 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra02227k |
|
| This journal is © The Royal Society of Chemistry 2014 |
Click here to see how this site uses Cookies. View our privacy policy here. ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 through simple mixing at 110 °C under stirring for 2 hours. Melting points were determined on a XRC-1 Microscopic Melting Point Measurer without correction. 1H NMR spectra were recorded on a Bruker AV-400 instrument at room temperature by using TMS as internal standard. For easy understanding, chemical structures for the cation of cholinium-based ionic liquids were shown in Scheme 1.
1 through simple mixing at 110 °C under stirring for 2 hours. Melting points were determined on a XRC-1 Microscopic Melting Point Measurer without correction. 1H NMR spectra were recorded on a Bruker AV-400 instrument at room temperature by using TMS as internal standard. For easy understanding, chemical structures for the cation of cholinium-based ionic liquids were shown in Scheme 1.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.05. The maximum absorbance of the enol tautomer of EAA is at 244 nm.
0.05. The maximum absorbance of the enol tautomer of EAA is at 244 nm.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.1
1.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and the reaction was conducted at 90 °C.
1, and the reaction was conducted at 90 °C.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) resorcinol)
resorcinol)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond of β-ketoesters would benefit to its reactivity. When it comes to EAA, the polarization change of C
O bond of β-ketoesters would benefit to its reactivity. When it comes to EAA, the polarization change of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O will affect its keto–enol tautomeric equilibrium. Therefore, we studied the effect of several ionic liquids with [HSO4] anion on the keto–enol tautomeric equilibrium of EAA in methanol and the results are illustrated in Fig. 1. It can be seen that when the selected ionic liquids were added to the methanol solution of EAA, absorbance of the enol decreased in the sequence of [choline][HSO4] > [N112][HSO4] > [N112OH][HSO4]. This suggests that the addition of ionic liquid will increase the keto content in the tautomeric equilibrium and ionic liquid [N112OH][HSO4], which has stronger acidity than [choline][HSO4] and a hydroxyl group on the cation, is the most favorable for the existence of keto. Therefore, a plausible reaction mechanism was proposed and illustrated in Fig. 2. It is suggested that the hydroxyl group on the cation of [N112OH][HSO4] acts as a hydrogen bond donor to polarize the carbonyl group of EAA and increase keto content of EAA. This makes the carbonyl group more susceptible to the subsequent nucleophilic attacks by resorcinol.
O will affect its keto–enol tautomeric equilibrium. Therefore, we studied the effect of several ionic liquids with [HSO4] anion on the keto–enol tautomeric equilibrium of EAA in methanol and the results are illustrated in Fig. 1. It can be seen that when the selected ionic liquids were added to the methanol solution of EAA, absorbance of the enol decreased in the sequence of [choline][HSO4] > [N112][HSO4] > [N112OH][HSO4]. This suggests that the addition of ionic liquid will increase the keto content in the tautomeric equilibrium and ionic liquid [N112OH][HSO4], which has stronger acidity than [choline][HSO4] and a hydroxyl group on the cation, is the most favorable for the existence of keto. Therefore, a plausible reaction mechanism was proposed and illustrated in Fig. 2. It is suggested that the hydroxyl group on the cation of [N112OH][HSO4] acts as a hydrogen bond donor to polarize the carbonyl group of EAA and increase keto content of EAA. This makes the carbonyl group more susceptible to the subsequent nucleophilic attacks by resorcinol.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.b The molar ratio of ionic liquid to phenol was 0.5
1.b The molar ratio of ionic liquid to phenol was 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.
1.