Synthesis of novel ferrocenyl N/O-heterocycles, chiral P,N-ligand and α-dehydro-β-amino acid derived short peptides from Morita–Baylis–Hillman adducts of ferrocenealdehyde†
Suchithra Madhavana,
Ponnusamy Shanmugam*b and
Ramavarma Luxmi Varmaa
aOrganic Chemistry Section, National Institute for Interdisciplinary Science and Technology (CSIR-NIIST), Trivandrum-695 019, India
bOrganic Chemistry Division, Central Leather Research Institute (CSIR-CLRI), Adyar, Chennai-600020, India. E-mail: shanmu196@rediffmail.com; Fax: +91-44-24911589; Tel: +91-044-24913289
First published on 28th May 2014
Abstract
The ‘golden triangle’ of Fc, OH/NH, COO moieties created by classical/aza-MBH reaction of ferrocenealdehyde has been exploited for the first time for the synthesis of novel multisubstituted ferrocenyl N/O heterocycles, chiral P,N ligands and ferrocenyl α-dehydro-β-peptides.
Ferrocene (Fc), the fascinating organometallic sandwich compound and its derivatives have received increasing interest from chemists due to their applications in asymmetric catalysis,1 materials chemistry,2 bio-organometallics3 and medicine.4 The unique structure of ferrocene is responsible for the wide variety of chiral ferrocenyl phosphine ligands, one of the most successful classes of ligands in asymmetric catalysis. Development of structurally innovative chiral ferrocene ligands for known asymmetric reactions and/or new applications from these ligands is a thriving area in synthetic organic chemistry. In the quest for novel hemilabile ligands, ferrocenyl pyrrolidines attained special attention which are proven efficient ortho-directing groups leading to the synthesis of chiral ferrocenyl P,N-ligands.5 Substituted dihydrofurans are key structural units in many natural products and also serve as useful synthetic intermediates.6 Hence, synthesis of multi substituted ferrocenyl N/O heterocycles is of high interest which will provide interesting scaffolds for the design of chiral ligands. Furthermore, ferrocene has recently been recognized as a reliable organometallic scaffold for its ability to induce secondary structures and supramolecular arrangements to its peptide conjugates. This bioorganometallic chemistry is envisioned to provide not only a peptidomimetic basis for protein folding, but also pharmacologically useful compounds, artificial receptors, asymmetric catalysts, new materials with functional properties, electrochemical sensor devices and immunoassay reagents.7
Stimulated by the lack of precedents for exploiting the ‘golden triangle’ of Fc, NH/OH, COO moieties of ferrocenyl Morita–Baylis–Hillman (MBH) adducts8 together with our ongoing interest in synthetic applications of MBH adducts9 we embarked upon the synthesis of ferrocenyl N/O heterocycles, chiral P,N-ligand and highly strained metallo β-peptides from MBH adducts of Fc-CHO and the results are presented in this communication.
Synthesis of ferrocenyl N/O heterocycles
The synthetic precursor's of ferrocenyl heterocycles viz. ferrocenyl MBH adducts 2–7 were prepared8,10 by classical and aza-MBH reaction of Fc-CHO, 1 (Scheme 1).Initially, ferrocenyl MBH adduct 2 on alkylation with K2CO3/allyl bromide afforded N-allylated adduct 8 in 88% yield. Ring closing metathesis (RCM) of 8 in toluene with 10 mol% Grubbs II generation catalyst yielded 2-ferrocenyl-3-cyano-pyrroline 11 in 48% yield. Similarly, ester derivatives of ferrocene appended pyrrolines 12 and 13 were also prepared from MBH adducts 3 and 4 in moderate yields (Scheme 2). On the other hand, the classical MBH adduct 7 underwent O-allylation followed by RCM to yield 2-ferrocenyl-2-cyano-dihydrofuran 16 in 40% yield.11 After the successful synthesis of ferrocenyl pyrroline and dihydrofuran derivatives, next we focussed on the synthesis of ferrocenyl piperidine derivative 14. Gratifyingly, [4 + 2]-annulation reaction12 of MBH adduct 4 with methyl vinyl ketone in presence of DBU afforded an inseparable diastereomeric mixture of tetrasubstituted ferrocenyl piperidine derivative 14 (dr. 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5) in 78% yield (Scheme 2).
0.5) in 78% yield (Scheme 2).
Synthesis of ferrocenyl P/N ligands
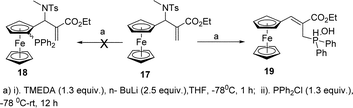
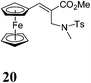
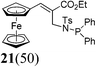
Next, keeping the goal of synthesis of structurally varied chiral ligands in mind, we investigated the directive orthometalating ability of NTs group attached to the ferrocene backbone of ferrocenyl MBH adducts. To our dismay, the lithiation of N-protected MBH adduct 17 with TMEDA and n-BuLi followed by quenching with phosphinyl chloride afforded the phosphine substituted product 19 instead of the expected acyclic chiral ligand 18 in 92% yield (Scheme 3). N-Allyl substitution in the MBH adduct 10 didn't alter the reaction which also yielded the phosphine substituted product 19 (Table 1, entry 1). Evidently, the rearranged MBH adduct 20 remained unaffected under the lithiation–phosphinylation condition (Table 1, entry 2).Metallation followed by phosphinylation reaction of unprotected MBH adduct 4, afforded rearranged N-phosphinylated product 21 along with 19 (Table 1, entry 3). The unprotected rearranged MBH adduct 6 also underwent same sort of reaction resulting into compounds 21 and 19 (Table 1, entry 4). On the basis of the above experiments, we concluded that planarity of Fc stabilises the rearranged product having NH moiety away from the Fc backbone, hence failed to direct the metallation to ortho position of cyclopentadiene ring, which jeopardized our efforts towards the synthesis of chiral ferrocenyl ligands. However, the method offers novel N-phosphinylated ferrocenyl derivatives in very good yield.
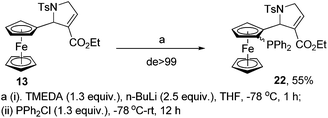
Then we turned our attention towards the ferrocenyl heterocycles, where the N/O pendant responsible for planar chiral induction is fixed in the cyclic framework attached to Fc-scaffold. The ferrocene matrix bearing pyrrolidine pendant 13 was chosen as the model substrate and its ability to undergo diastereoselective ortholithiation–phosphinylation was first investigated. Thus, treatment of THF solution of 13 at −78 °C with TMEDA and n-BuLi followed by quenching with phosphinyl chloride (−78 °C–rt) afforded novel planar and central chiral (racemic) ferrocenyl P,N ligand 22 in moderate yield with excellent diastereoselectivity (de > 99) (Scheme 4).
The structure of chiral ferrocenyl ligand 22 was established by spectroscopic (1H NMR, IR and Mass), multinuclear (13C{1H}, 31P{1H}) and 2DNMR techniques. The 1H NMR spectrum clearly showed signals for the unsubstituted cyclopentadienyl protons as a singlet for five protons at δ 4.20 ppm and 1,2-disubstituted cyclopentadienyl protons as three mutually coupled multiplets at δ 4.17–4.11, 3.71–3.49 and 3.28–3.19 ppm. Interestingly, the ester methylene protons appeared as two well separated multiplets due to the interaction with phosphine moiety. The 13C{1H}NMR spectra confirmed the structure by combining the signals of the PPh2 substituted ferrocene unit and pyrrolidine pendant with C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O resonances δC 172.4 ppm for ester carbonyl and alkene carbons at δC 136.1, 88.4 ppm, respectively. Finally, the 31P{1H}NMR spectrum displayed a resonance at δP −16.17 ppm.
O resonances δC 172.4 ppm for ester carbonyl and alkene carbons at δC 136.1, 88.4 ppm, respectively. Finally, the 31P{1H}NMR spectrum displayed a resonance at δP −16.17 ppm.
Synthesis of ferrocenyl-α-dehydro-β-peptides and β-lactam
α-Dehydro amino acids are important precursors of unnatural peptides that are capable to induce β-bends in small peptide sequences with enhanced biological activities and selectivity.13 Till now there is no attempt to synthesise stereochemically constrained dehydro-β-amino acid residues incorporating an organometallic scaffold such as ferrocene. In this scenario, we prepared two types of ferrocenyl α-dehydro-β-amino acids 23 and 24 by hydrolysis of the ferrocenyl MBH adduct 4 and rearranged adduct 6 under basic condition. During the hydrolysis of rearranged amino ester 6, along with acid 24 detosylated amine 25 was also obtained in 78% and 20% yields, respectively (Scheme 5).The α-dehydro-β-amino acid 24 was converted into dipeptide 26 with glycine ester hydrochloride by solution phase coupling reaction using EDC as coupling agent (Scheme 6).
For maximum use the conformational constrain exerted by the dehydro residue, L-proline having a constrained backbone dihedral angle has been utilized to prepare the corresponding short peptide 27 (Table 2, entry 1). Similarly, dehydro ferrocenyl amino acid 23 with glycine and L-proline yielded dipeptides 28 and 29, respectively (Table 2, entries 2 and 3). The synthetic potential of β-amino acid 23 to yield ferrocenyl β-lactam 30 was demonstrated by reacting 23 in THF with coupling agent bis(2-oxo-3-oxazolidinyl) phosphinic chloride (BOPCl) at room temperature (Table 2, entry 4). Indeed, these simple easy to prepare MBH derived strained ferrocenyl-β-aminoacids can be coupled with PNA's and biogenic peptides like enkephalin and bradykinin analogues for organometallic labelling like Sonogashira and click reaction.3a,14
| Entry | Fc-amino acid | Amino acid | Dipeptide | Yield (%) |
|---|---|---|---|---|
a Amino acid (1 equiv.), EDC·HCl (3 equiv.), HOBt·H2O (3.5 equiv.), DIPEA (5 equiv.), DCM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMF (1 DMF (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), 0 °C–rt, 12 h.b BOPCl (1.5 equiv.), DIPEA (1.5 equiv.), THF, rt, 12 h. 1), 0 °C–rt, 12 h.b BOPCl (1.5 equiv.), DIPEA (1.5 equiv.), THF, rt, 12 h. |
||||
| 1 |  |
HCl·H-(L)Pro-OMe |  |
88a |
| 2 |  |
HCl·H-Gly-OMe |  |
84a |
| 3 | 23 | HCl·H-(L)Pro-OMe |  |
86a |
| 4 | 23 | 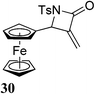 |
70b | |
Conclusions
In conclusion, the synthesis of novel multisubstituted ferrocenyl pyrrolidines, furan and piperidine from MBH adducts of ferrocenealdehyde have been achieved. Ferrocenyl P,N ligand with multiple chirality has been synthesised involving a highly diastereoselective ortholithiation (de > 99), adding new class of privileged ligands to the current repertoire. A short synthesis of novel ferrocenyl α-dehydro-β-peptides, a new entry for de novo peptide design has also been reported herein. Efforts to synthesise and study the catalytic activity of analogues ligands from ferrocenyl MBH adducts are in progress.Acknowledgements
PS thanks the Directors of NIIST and CLRI for providing infrastructure facilities. SM (NIIST) thanks CSIR (New Delhi) for the award of SRF. Financial support from CSIR 12th five year project CSC 0201 is acknowledged. Thanks are due to Mrs Viji and Mr Adarsh B for recording mass and NMR spectra, respectively.Notes and references
- (a) P. Stepnicka, Ferrocenes: Ligands, Materials and Biomolecules, John Wiley & Sons, New York, 2008 Search PubMed; (b) R. C. J. Atkinson, V. C. Gibson and N. J. Long, Chem. Soc. Rev., 2004, 41, 313 RSC; (c) L.-X. Dai and X.-L. Hou, Chiral Ferrocenes in Asymmetric Catalysis: Synthesis and Applications, Wiley-VCH, Weinheim, 2010 Search PubMed; (d) S. L. Marquard, D. C. Rosenfeld and J. F. Hartwig, Angew. Chem., Int. Ed., 2010, 49, 793 CrossRef CAS PubMed; (e) R. G. Arrayas, J. Adrio and J. C. Carretero, Angew. Chem., Int. Ed., 2006, 45, 7674 CrossRef PubMed.
- (a) D. Astruc, New J. Chem., 2011, 35, 764 RSC; (b) C. Jin, J. Lee, E. Lee, E. Hwang and H. Lee, Chem. Commun., 2012, 48, 4235 RSC; (c) H. Wang, N. Yan, Y. Li, X. Zhou, J. Chen, B. Yu, M. Gong and Q. Chen, J. Mater. Chem., 2012, 22, 9230 RSC; (d) H. Tiana and L. Sun, J. Mater. Chem., 2011, 21, 10592 RSC; (e) H. Kumari, C. L. Dennis, A. V. Mossine, C. A. Deakyne and J. L. Atwood, J. Am. Chem. Soc., 2013, 135, 7110 CrossRef CAS PubMed; (f) M. Ortiz, E. M. Wajs, A. Fragoso and C. K. O'Sullivan, Chem. Commun., 2012, 48, 1045 RSC; (g) M. C. Martos-Maldonado, M. B. Thygesen, K. J. Jensen and A. Vargas-Berenguel, Eur. J. Org. Chem., 2013, 2793 CrossRef CAS; (h) M. Nakahata, Y. Takashima, A. Hashidzume and A. Harada, Angew. Chem., Int. Ed., 2013, 52, 5731 CrossRef CAS PubMed.
- (a) D. R. van Staveren and N. Metzler-Nolte, Chem. Rev., 2004, 104, 5931 CrossRef CAS PubMed; (b) E. Katz and I. Willner, Angew. Chem., Int. Ed., 2004, 43, 6042 CrossRef CAS PubMed; (c) C. G. Hartinger and P. J. Dyson, Chem. Soc. Rev., 2009, 38, 391 RSC.
- (a) C. Ornelas, New J. Chem., 2011, 35, 1973 RSC; (b) B. Kater, A. Hunold, H.-G. Schmalz, L. Kater, B. Bonitzki, P. Jesse and A. Prokop, J. Cancer Res. Clin. Oncol., 2011, 137, 639 CrossRef CAS PubMed; (c) P. F. Salas, C. Herrmann, J. F. Cawthray, C. Nimphius, A. Kenkel, J. Chen, C. de Kock, P. J. Smith, B. O. Patrick, M. J. Adam and C. Orvig, J. Med. Chem., 2013, 56, 159 CrossRef PubMed; (d) K. Kerman and H.-B. Kraatz, Analyst, 2009, 134, 2400 RSC.
- (a) T. Ahern, H. Muller-Bunz and P. J. Guiry, J. Org. Chem., 2006, 71, 7596 CrossRef CAS PubMed; (b) M. Soueidan, M. Jida, T. Bousquet, F. A. Niedercorna and L. Pelinski, New J. Chem., 2011, 35, 991 RSC; (c) C.-M. Liu, W.-Y. Liu, Y.-M. Liang and Y.-X. Ma, Synth. Commun., 2000, 30, 1755 CrossRef CAS; (d) P. Vicennati and P. G. Cozzi, Eur. J. Org. Chem., 2007, 2248 CrossRef CAS; (e) A. Farrell, R. Goddard and P. J. Guiry, J. Org. Chem., 2002, 67, 4209 CrossRef CAS PubMed.
- (a) B. Figadere, Acc. Chem. Res., 1995, 28, 359 CrossRef CAS; (b) R. Shen, S. Zhu and X. Huang, J. Org. Chem., 2009, 74, 4118 CrossRef CAS PubMed; (c) Q.-F. Wang, H. Hou, L. Hui and C.-G. Yan, J. Org. Chem., 2009, 74, 7403 CrossRef CAS PubMed; (d) X. Dou, F. Zhong and Y. Lu, Chem.–Eur. J., 2012, 18, 13945 CrossRef CAS PubMed; (e) C. Zhong, T. Liao, O. Tuguldur and X. Shi, Org. Lett., 2010, 12, 2064 CrossRef CAS PubMed; (f) M. Tiecco, L. Testaferri and C. Santi, Eur. J. Org. Chem., 1999, 797 CrossRef CAS.
- (a) T. Moriuchi and T. Hirao, Chem. Soc. Rev., 2004, 33, 294 RSC; (b) S. Martic, M. Labib, P. O. Shipman and H.-B. Kraatz, Dalton Trans., 2011, 40, 7264 RSC; (c) T. Moriuchi and T. Hirao, Acc. Chem. Res., 2010, 43, 2010 CrossRef PubMed; (d) D. Siebler, C. Forster and K. Heinze, Dalton Trans., 2011, 40, 3558 RSC; (e) C.-W. Wei, Y. Peng, L. Zhang, Q. Huang, M. Cheng, Y.-N. Liu and J. Li, Bioorg. Med. Chem. Lett., 2011, 21, 5818 CrossRef CAS PubMed; (f) Y. Arikuma, H. Nakayama, T. Morita and S. Kimura, Angew. Chem., Int. Ed., 2010, 49, 1800 CrossRef CAS PubMed; (g) S. R. Beeren and J. K. M. Sanders, J. Am. Chem. Soc., 2011, 133, 3804 CrossRef CAS PubMed.
- S. Madhavan and P. Shanmugam, Org. Lett., 2011, 13, 1590 CrossRef CAS PubMed.
- (a) B. Viswambharan, K. Selvakumar, S. Madhavan and P. Shanmugam, Org. Lett., 2010, 12, 2108 CrossRef CAS PubMed; (b) R. Solaiselvi, P. Shanmugam and A. B. Mandal, Org. Lett., 2013, 15, 1186 CrossRef CAS PubMed.
- (a) K. Senthil Kumar and K. C. Kumara Swamy, J. Organomet. Chem., 2001, 637–639, 616 CrossRef; (b) P. Shanmugam, V. Vaithiyanathan, B. Viswambharan and S. Madhavan, Tetrahedron Lett., 2007, 48, 9190 CrossRef CAS PubMed.
- (a) J. M. Kim, K. Y. Lee, S. Lee and J. N. Kim, Tetrahedron Lett., 2004, 45, 2805 CrossRef CAS PubMed; (b) H. S. Lee, J. M. Kim and J. N. Kim, Tetrahedron Lett., 2007, 48, 4119 CrossRef CAS PubMed; (c) B. Schmidt, Eur. J. Org. Chem., 2003, 816 CrossRef CAS; (d) D. Balan and H. Adolfsson, Tetrahedron Lett., 2004, 45, 3089 CrossRef CAS PubMed.
- D. Y. Park, M. J. Lee, T. H. Kim and J. N. Kim, Tetrahedron Lett., 2005, 46, 8799 CrossRef CAS PubMed.
- (a) P. Mathur, S. Ramakumar and V. S. Chauhan, Biopolymers, 2004, 76, 150 CrossRef CAS PubMed; (b) R. Ramapanicker, R. Mishra and S. Chandrasekaran, J. Pept. Sci., 2010, 16, 123 CAS; (c) G. Cardillo, A. Gennari, L. Gentilucci, E. Mosconi, A. Tolomelli and S. Troisi, Eur. J. Org. Chem., 2009, 5991 CrossRef CAS; (d) S. Rajesh, B. Banerji and J. Iqbal, J. Org. Chem., 2002, 67, 7852 CrossRef CAS PubMed; (e) M. Gupta, A. Bagaria, A. Mishra, P. Mathur, A. Basu, S. Ramakumar and V. S. Chauhan, Adv. Mater., 2007, 19, 858 CrossRef CAS.
- (a) B. Zhou, C.-L. Li, Y. Hao, M. C. Johnny, Y. A. N. Lui and J. Li, Bioorg. Med. Chem., 2013, 21, 395 CrossRef CAS PubMed; (b) S. Maricic, U. Berg and T. Frejd, Tetrahedron, 2003, 58, 3085 CrossRef; (c) A. Pinto, U. Hoffmanns, M. Ott, G. Fricker and N. Metzler-Nolte, ChemBioChem, 2009, 10, 1852 CrossRef CAS PubMed; (d) S. D. Köster, J. Dittrich, G. Gasser, N. Hüsken, I. C. H. Castañeda, J. L. Jios, C. O. D. Védova and N. Metzler-Nolte, Organometallics, 2008, 27, 6326 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures and spectral details of the products. See DOI: 10.1039/c4ra02201g |
| This journal is © The Royal Society of Chemistry 2014 |