Pd/C-catalyzed cross-coupling reaction of benzyloxysilanes with halosilanes for selective synthesis of unsymmetrical siloxanes†
Abstract
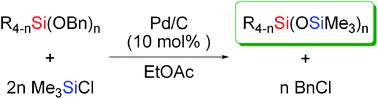
A new protocol for the nonhydrolytic synthesis of unsymmetrical siloxanes has been developed. The cross-coupling reaction of benzyloxysilanes with halosilanes catalyzed by Pd/C afforded various unsymmetrical siloxanes with co-production of benzyl halides.

 Please wait while we load your content...
Please wait while we load your content...