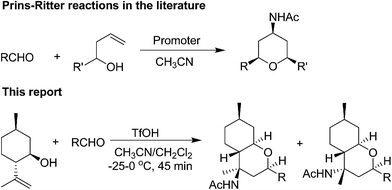
First example of a Prins–Ritter reaction on terpenoids: a diastereoselective route to novel 4-amido-octahydro-2H-chromenes†
Barnali Sarmaha,
Gakul Baishya*a and
Rajani K. Baruahb
aNatural Products Chemistry Division, CSIR-North East Institute of Science & Technology, Jorhat-785006, India. E-mail: b.gakul@gmail.com; Fax: +91-376-2370011
bAnalytical Chemistry Division, CSIR-North East Institute of Science & Technology, Jorhat-785006, India. Fax: +91-376-2370011
First published on 29th April 2014
Abstract
(−)-Isopulegol was subjected to a triflic acid-promoted three-component Prins–Ritter reaction with a series of aldehydes to produce a library of novel 4-acetamido-octahydro-2H-chromene derivatives in good yields and high diastereoselectivities.
Introduction
The highly functionalized tetrahydropyran ring, the key structural motif of many biologically and synthetically important natural products, has attracted a great deal of attention in the field of synthetic organic chemistry.1 The construction of tetrahydropyran rings is generally achieved in a single step chemical process via the well-known Prins cyclization reaction of homoallylic alcohols with aldehydes or ketones.2 Recently, one-pot, three-component reactions have shown their potential in various applications of pharmaceutical chemistry such as the production of structural scaffolds and in combinatorial libraries for drug discovery.3 The Prins cyclization method could be utilized as an initiator of a tandem three-component reaction because the carbocation formed during the course of the reaction has to be quenched with various nucleophiles.4 Moreover, the technique of a tandem reaction is also a valuable synthetic tool that plays an important role in the synthesis of natural product-like molecules.5 The major advantage of a tandem reaction is the consecutive formation of several covalent bonds, including C–C, C–O, and C–N, among others, by a single catalyst in one-pot. Again, amino tetrahydropyrans are the core structures of many natural products such as ambrucitin VS, oligomer of glycamino acids, sialic acid and dysiherbaine, etc.7 Various applications8 of these compounds in photographic plates as well as in host–guest chemistry are well documented in the literature. The sequence of the Prins–Ritter reaction could be utilized as the best synthetic method to build the six membered ring of 4-amino tetrahydropyran derivative efficiently in a single step reaction (Scheme 1).6 It is also well established that the Prins–Ritter reaction sequence can be efficiently utilized for the synthesis of optically pure natural alkaloids (−)-halosaline and (−)-norallosedamine without loss of their optical purities.6Perron and Albizati first developed a tandem Prins–Ritter sequence wherein 4-acetamido-pyranosides were synthesized through a SnCl4 mediated reaction of orthoesters with homoallylic alcohols in acetonitrile.4e Willis and co-workers also reported the Prins–Ritter tandem cyclization of acetal of a homoallylic alcohol in the presence of triflic acid in acetonitrile.9 Recently, Yadav et al. also demonstrated the tandem Prins–Ritter reaction of homoallylic alcohols, aldehydes or ketones and nitriles in two different reports using a catalytic mixture CeCl3–acetyl chloride10a and phosphomolybdic acid.10b The Sakurai–Prins–Ritter reaction sequence was also efficiently employed to synthesize synthetically important 4-acylamino-2,6-disubstituted tetrahydropyran derivatives.11 Again, 2H-chromene12 is also found to be the core structural motif of several biologically active natural products such as calonolide F, which was isolated from Calophyllum teysmannii.13 Calonolide F mainly exhibits anti-HIV activity, whereas its synthetic analogues show anti-hypertensive14 and anti-ischaemic15 activities. Although there are many existing reports on Prins–Ritter16 and Sakurai–Prins–Ritter reactions in the literature, to the best of our knowledge, this is the first example of the synthesis of novel 4-acetamido-octahydro-2H-chromene derivatives via the Prins–Ritter reaction of (−)-isopulegol with aldehydes.
As a part of our ongoing research programme on the Prins cyclization reaction,17 we report herein the successful execution of the Prins–Ritter strategy for the synthesis of a library of novel 4-acetamido-octahydro-2H-chromene derivatives from (−)-isopulegol and aldehydes using triflic acid as a promoter under very mild conditions (Scheme 1, This report).
Results and discussions
In search of optimal reaction conditions, we first attempted the reaction of (−)-isopulegol (1 mmol) with p-anisaldehyde (1.2 mmol) using 1 equivalent of triflic acid (1 mmol) as a promoter in acetonitrile at 24 °C. But the reaction gave a complex mixture of products. Repeating the reaction at 0 °C as well as −10 °C failed to control the formation of by-products. As such, we considered performing the reaction at a lower temperature to address the problem of formation of multiple products. Thus, we performed the same reaction at −20 °C using one equivalent of triflic acid. This time, the reaction proceeded smoothly affording a diastereomeric mixture of Prins–Ritter products 3c and 4c, as confirmed by their 1H NMR spectra, along with the diastereomeric mixture of the normal Prins cyclized products, 5c and 6c. However, the yield of the reaction was very poor (37%). To obtain a better yield, we also increased the amount of TfOH to 1.5, 2.0 and 3.0 equivalents to obtain the diastereomeric mixture of Prins–Ritter products in 53%, 72% and 70% yields, respectively. However, 10–13% yield of the normal Prins cyclized product (5c and 6c) was also isolated in each case. In subsequent experiments, we also tried to stop the formation of the normal Prins cyclized product by decreasing the temperature to −25 °C, −40 °C and −50 °C using 2.0 equivalent of triflic acid. We found that the formation of the normal Prins cyclized product could not be completely avoided and 8–9% yield of 5c and 6c were also obtained in each case. Thus, the optimal reaction conditions involved the use of (−)-isopulegol 1 (1 mmol) with p-anisaldehyde 2c (1.2 mmol) and a solution of 2 equivalents of triflic acid (2 mmol) as the promoter in 1 mL CH2Cl2 in acetonitrile (1 mL) at −25 °C (Table 1).| Entry | TfOH (equiv.) | Temperature | Time | Yieldb (%) 3c + 4c | Yieldb (%) 5c + 6c |
|---|---|---|---|---|---|
a Reaction conditions: reaction performed with 1.2 mmol of 2c and 1 mmol of 1 at a1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of CH3CN/CH2Cl2 in 2 mL solution.b Yields are for isolated products as mixtures. 1 ratio of CH3CN/CH2Cl2 in 2 mL solution.b Yields are for isolated products as mixtures. |
|||||
| 1 | 1.0 | 24 °C | 1 h | — | — |
| 2 | 1.0 | 0 °C | 1 h | — | — |
| 3 | 1.0 | −10 °C | 1 h | — | — |
| 4 | 1.0 | −20 °C | 45 min | 37 | 12 |
| 5 | 1.5 | −20 °C | 45 min | 53 | 13 |
| 6 | 2.0 | −20 °C | 45 min | 72 | 10 |
| 7 | 3.0 | −20 °C | 45 min | 70 | 14 |
| 8 | 2.0 | −25 °C | 45 min | 76 | 7 |
| 9 | 2.0 | −40 °C | 45 min | 76 | 8 |
| 10 | 2.0 | −50 °C | 45 min | 74 | 7 |
After optimization of the reaction conditions, we explored the general applicability and scope of this protocol with various aromatic and aliphatic aldehydes; the results are presented in the Table 2.
| Entry | Aldehyde R= | Product 3a | Product 4a | % Yieldb | Ratio 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4c 4c |
|---|---|---|---|---|---|
| a Products 3 and 4 were characterized by their 1H NMR, 13C NMR, mass and IR spectra.b Combined isolated yields.c Diastereomeric ratios were obtained from their isolated yields.d Diasteromeric ratio was obtained from its 1HNMR spectrum.e Major isomer 3n was exclusively obtained and 4n was not isolated in a trace amount. | |||||
| a | Ph |  |
 |
62 | 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| b | 2-Naphthyl |  |
 |
68 | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| c | 4-MeO-Ph |  |
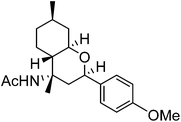 |
76 | 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| d | 4-Me-Ph | 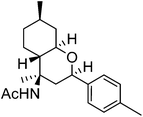 |
 |
72 | 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| e | 4-iso-Propyl-Ph |  |
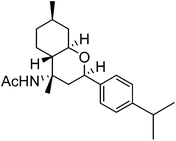 |
76 | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| f | 3-NO2-Ph |  |
 |
64 | 3.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1d 1d |
| g | 4-Cl-Ph |  |
 |
70 | 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| h | 3,4-Cl2-Ph |  |
 |
60 | 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| i | 4-Br-Ph | 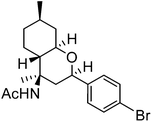 |
 |
62 | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| j | 2-Furfuryl | 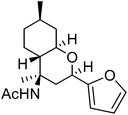 |
 |
67 | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| k | 2-Thiophenyl | 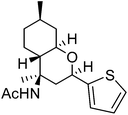 |
 |
64 | 3.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| l | n-Ethyl |  |
 |
60 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| m | 3-Phenyethyl |  |
 |
62 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| n | iso-Propyl |  |
58 | —e | |
| o | Cyclohexyl |  |
 |
65 | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
The exploration of this Prins–Ritter protocol began with the reaction of (−)-isopulegol with various aromatic and heteroaromatic aldehydes. Various substituted aromatic aldehydes including p-tolualdehyde, p-isopropylbenzaldehyde, m-nitrobenzaldehyde, p-chlorobenzaldehyde, 3,4-dichlorobenzaldehyde and p-bromobenzaldehyde underwent the Prins–Ritter reaction smoothly to produce their corresponding 4-acetamido-octahydro-2H-chromene derivatives under the optimized reaction conditions (entries d–i, Table 2). The crude products were purified by column chromatography to afford pure 4-acetamido-octahydro-2H-chromenes. However, the 1H NMR spectra of the pure products after column chromatography revealed the presence of a mixture of two diastereomers. In order to separate the two diastereomers, we performed a re-crystallization and successfully separated both species as optically pure diastereomers. Both aromatic aldehydes bearing electron donating and electron withdrawing substituents afforded 4-acetamido-octahydro-2H-chromene derivatives in good yields; however, the ratios of diastereomers obtained after re-crystallization were higher in the case of aromatic aldehydes with electron donating substituents. Unsubstituted aromatic aldehydes such as benzaldehyde and 2-napthaldehyde also underwent a Prins–Ritter reaction to afford their corresponding 4-acetamido-octahydro-4H-chromene in good yields and good diastereoselectivities (entries a & b, Table 2). Interestingly, the Prins–Ritter reaction of heteroaromatic aldehydes including 2-furfuraldehye and 2-thiophene carbaldehyde with (−)-isopulegol under the same reaction condition also afforded their corresponding acetamido chromene derivatives in good yields and good diastereoselectivities (entries j & k, Table 2).
To confirm the utility of this protocol, various aliphatic aldehydes were also reacted with (−)-isopulegol and acetonitrile under the same reaction conditions. For example, propanal, 3-phenyl propanal, cyclohexyl carbaldehyde and isobutaraldehyde also underwent a smooth Prins–Ritter reaction with (−)-isopulegol and acetonitrile in the presence of 2 equivalents of triflic acid at −25 °C to yield their corresponding 4-acetamido-octahydro-2H-chromenes (entries l–o, Table 2). Surprisingly, the products were also obtained in good yields and good diastereoselectivities using these aldehydes. However, in the case of aliphatic aldehydes, the diastereomers produced in each reaction were separated by simple silica gel column chromatography.
Furthermore, it has been established that this methodology can be extended to conjugated aldehydes and works well with cinnamaldehyde to furnish the corresponding 4-acetamido-octahydro-2H-chromenes 7 and 8 in moderate yield and good diastereoselectivity (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 = 4
8 = 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (Scheme 2). Like other aliphatic aldehydes, the diastereomers obtained in this case were also separated by silica gel column chromatography.
1) (Scheme 2). Like other aliphatic aldehydes, the diastereomers obtained in this case were also separated by silica gel column chromatography.
In subsequent experiments, we also explored the addition of diversity at the 4-position of the chromene derivatives using different nitrile molecules. Thus, different nitrile molecules including benzonitrile, acrylonitrile, piperonitrile were reacted with (−)-isopulegol and p-anisaldehyde in three different reactions. We found that only benzonitrile underwent the Prins–Ritter sequence to afford the corresponding 4-benzamido-octahydro-2H-chromene derivatives 9 and 10 in good yield. In addition, the diastereoselectivity could not be achieved from the isolated yield because isomer 9 could not be isolated in its pure form by normal silica gel column chromatography (Scheme 3).
A possible mechanism for this important transformation has been proposed in Scheme 4. It has been shown that the aldehyde group is first protonated by the triflic acid that has been attacked by the hydroxyl group of the isopulegol molecule. The subsequent proton transfer followed by the removal of a water molecule leads to the oxocarbenium ion A that undergoes a Prins cyclization via its common cis-selective pathway to produce the tetrahydropyranyl tertiary carbocation B. The nucleophile (acetonitrile) present in the reaction medium attacks the carbocation B from either sides to give the intermediates C and D, which under hydrolysis give the desired products. The major isomer possesses the equatorial acetamide group, which was confirmed by the single-X-ray crystallography of 3c (Fig. 1) and NOESY experiment of 4c. These results indicate that the trapping of the carbocation B favors the equatorial side.
Conclusion
In conclusion, an operationally simple method has been developed for the diastereoselective synthesis of novel 4-acetamido-octahydro-2H-chromene derivatives using a one-pot sequential Prins–Ritter reaction of (−)-isopulegol with aldehydes in the presence of triflic acid as the promoter under very mild reaction conditions. A wide range of non-substituted and substituted aromatic aldehydes with both electron donating and electron withdrawing substituents underwent a Prins–Ritter reaction with (−)-isopulegol. This protocol was also equally effective with various aliphatic and conjugated aldehydes. The triflic acid promoted Prins–Ritter reaction offers a new synthetic route for the synthesis of novel 4-amido-octahydro-2H-chromene derivatives in a single step.Experimental
General methods
Melting points were measured using a Buchi B-540 melting point apparatus and are uncorrected. IR spectra were recorded on a SHIMADZU FTIR-8400. NMR spectra were recorded on a Bruker DPX 300 MHz, AV500 Advance-III 500 MHz and Jeol JNM 400 MHz spectrometers using tetramethylsilane (TMS) as an internal standard. Mass spectra were recorded on an ESQUIRE 3000 Mass spectrometer. All the commercially available reagents were used without further purification. All experiments were monitored by thin layer chromatography using aluminum pre-coated silica gel TLC plates (Merck). After elution, the spots were visualized under UV illumination at 254 nm. Further visualization was achieved by staining the anisaldehyde charring solution. Column chromatography was performed on silica gel (100–200 mesh, Rankem) using an appropriate ethyl acetate–hexane mixture. Specific rotation values were measured on a PerkinElmer Polarimeter model 343.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 ethyl acetate–hexane as the eluent to obtain the Prins–Ritter products 3 and 4 (as diastereomeric mixture as confirmed by its 1H NMR spectrum). The two diastereomers were separated from the mixture by re-crystallization from hexane. The structure of compounds 5c and 6c was confirmed by comparing their analytical data with those reported in the literature.17a
7 ethyl acetate–hexane as the eluent to obtain the Prins–Ritter products 3 and 4 (as diastereomeric mixture as confirmed by its 1H NMR spectrum). The two diastereomers were separated from the mixture by re-crystallization from hexane. The structure of compounds 5c and 6c was confirmed by comparing their analytical data with those reported in the literature.17aAcknowledgements
We are grateful to Department of Science & Technology, New Delhi, for financial support to this work and for fellowship of BS. We thank the Director, CSIR-NEIST, Jorhat, for his keen interest and encouragement.References
- Y. J. Class and P. DeShong, Chem. Rev., 1995, 95, 1843 CrossRef CAS; R. D. Norcross and I. Paterson, Chem. Rev., 1995, 95, 2041 CrossRef; I. E. Markó and D. J. Bayston, Synthesis, 1996, 297 CrossRef PubMed; S. D. Rychnovsky, G. Yang, Y. Hu and U. D. Khire, J. Org. Chem., 1997, 62, 3022 CrossRef; A. B. Smith, III, P. R. Verhoest, K. P. Minbiole and M. Schelhaas, J. Am. Chem. Soc., 2001, 123, 4834 CrossRef; D. J. Kopecky and S. D. Rychnovsky, J. Am. Chem. Soc., 2001, 123, 8420 CrossRef; Y. Wang, J. Janjic and S. A. Kozmin, J. Am. Chem. Soc., 2002, 124, 13670 CrossRef PubMed; D. L. Aubele, S. Wan and P. E. Floreancig, Angew. Chem., Int. Ed., 2005, 44, 3485 CrossRef PubMed; X.-F. Yang, M. Wang, Y. Zhang and C.-J. Li, Synlett, 2005, 1912 Search PubMed; X. Tian, J. J. Jaber and S. D. Rychnovsky, J. Org. Chem., 2006, 71, 3176 CrossRef PubMed; K. B. Bahnck and S. D. Rychnovsky, Chem. Commun., 2006, 2388 RSC; K. C. Nicolaou and E. J. Sorensen, Classics in Total Synthesis, VCH, Weinham, 1996 Search PubMed.
- (a) E. Arundale and L. A. Mikeska, Chem. Rev., 1952, 51, 505–555 CrossRef CAS; (b) B. B. Snider, in Comprehensive Organic Synthesis, ed. B. M. Trost, Pergamon Press, New York, 1991, vol. 2, p. 527 Search PubMed; (c) C. Olier, M. Kaafarani, S. Gastaldi and M. P. Bertrand, Tetrahedron, 2010, 66, 413–445 CrossRef CAS PubMed.
- Reviews on multi-component reactions: (a) L. F. Tietze and U. Beifuss, Angew. Chem., Int. Ed., 1993, 32, 131 CrossRef; (b) L. F. Tietze, Chem. Rev., 1996, 96, 115 CrossRef CAS PubMedL. F. Tietze and N. Rackelmann, Pure Appl. Chem., 2004, 76, 1967 CrossRef CAS; (c) A. Padwa, Pure Appl. Chem., 2004, 76, 1933 CrossRef CASJ. Zhu and H. Bienayme, Multicomponent Reactions, Wiley, Weinheim, 2005 Search PubMed.
- (a) S. D. Rychnovsky and C. R. Thomas, Org. Lett., 2000, 2, 1217 CrossRef CAS; (b) X.-F. Yang, M. Wang, Y. Zhang and C.-J. Li, Synlett, 2005, 1912 CAS; (c) O. L. Epstein and T. Tomislav Rovis, J. Am. Chem. Soc., 2006, 128, 16480 CrossRef CAS PubMed; (d) J. S. Yadav, B. V. Subba Reddy, T. Maity and G. G. K. S. Narayana Kumar, Tetrahedron Lett., 2007, 48, 7155 CrossRef CAS PubMed; (e) Z. Y. Wei, J. S. Li, D. Wang and T. H. Chan, Tetrahedron Lett., 1987, 28, 3441 CrossRef CAS; (f) F. Perron and K. F. J. Albizati, Org. Chem., 1987, 52, 4130 CrossRef; (g) Z. Y. Wei, D. Wang, J. S. Li and T. H. J. Chan, Org. Chem., 1989, 54, 5768 CrossRef CAS; (h) L. Coppi, A. Ricci and M. J. Taddei, Org. Chem., 1988, 53, 913 CrossRef; (i) G. S. Viswanathan, J. Yang and C. J. Li, Org. Lett., 1999, 1, 993 CrossRef CAS; (j) J. S. Yadav, B. V. Subba Reddy, G. G. K. S. Narayana Kumar and T. Swamy, Tetrahedron Lett., 2007, 48, 2205 CrossRef CAS PubMed.
- (a) G. H. Posner, Chem. Rev., 1986, 86, 831 CrossRef CAS; (b) T.-L. Ho, Tandem Organic Reactions, Wiley-Interscience, New York, 1992 Search PubMed; (c) N. Hall, Science, 1994, 266, 32 CAS; (d) L. F. Tietze, Chem. Rev., 1996, 96, 115 CrossRef CAS PubMed; (e) J. Montgomery, Angew. Chem., Int. Ed., 2004, 43, 3890 CrossRef CAS PubMed; (f) E. Negishi, C. Coperet, S. Ma, S. Y. Liou and F. Liu, Chem. Rev., 1996, 96, 365 CrossRef CAS PubMed; (g) T. Miura and M. Murakami, Chem. Commun., 2007, 217 RSC; (h) K. Agapiou, D. F. Cauble and M. J. Krische, J. Am. Chem. Soc., 2004, 126, 4528 CrossRef CAS PubMed; (i) K. Subburaj and J. Montgomery, J. Am. Chem. Soc., 2003, 125, 11210 CrossRef CAS PubMed; (j) H. C. Guo and J. A. Ma, Angew. Chem., Int. Ed., 2006, 45, 354 CrossRef CAS PubMed; (k) D. Enders, M. R. M. Huttl, C. Grondal and G. Raabe, Nature, 2006, 441, 861 CrossRef CAS PubMed; (l) S. Cabrera, J. Alemen, P. Bolze, S. Bertelsen and K. A. Jorgensen, Angew. Chem., Int. Ed., 2008, 47, 121 CrossRef CAS PubMed; (m) O. Penon, A. Carlone, A. Mazzanti, M. Locatelli, L. Sambri, G. Bartoli and P. Melchiorre, Chem.–Eur. J., 2008, 14, 4788 CrossRef CAS PubMed.
- J. S. Yadav, Y. Jayasudhan Reddy, P. Adi Narayana Reddy and B. V. Subba Reddy, Org. Lett., 2013, 15, 546 CrossRef CAS PubMed , and references cited therein.
- (a) V. Michelet and J.-P. Genet, Curr. Org. Chem., 2005, 9, 405 CrossRef CAS; (b) G. Hoefle, H. Steinmetz, K. Gerth and H. Reichenbach, Liebigs Ann. Chem., 1991, 941 CrossRef CAS; (c) Y. Suhara, Y. Yamaguchi, B. Collins, R. L. Schnaar, M. Yanagishita, J. E. K. Hildreth, I. Shimada and Y. Ichikawa, Bioorg. Med. Chem., 2002, 10, 1999 CrossRef CAS; (d) A. Dondoni, A. Boscarato and A. Marra, Tetrahedron: Asymmetry, 1994, 5, 2209 CrossRef CAS; (e) S. Ciccotosto and M. von Itzstein, Tetrahedron Lett., 1995, 36, 5405 CAS; (f) S. Sabesan, S. Neira and Z. Wasserman, Carbohydr. Res., 1995, 267, 239 CrossRef CAS; (g) B. B. Snider and N. A. Hawryluk, Org. Lett., 2000, 2, 635–638 CrossRef CAS PubMed.
- (a) H. Sakata and H. Yasukawa, Jpn. Kokai Tokkyo Koho, 2003, 42 Search PubMed; (b) G. J. McGarvey, M. W. Stepanian, A. R. Bressette and M. Sabat, Org. Lett., 2000, 2, 3453 CrossRef CAS PubMed.
- E. H. Al-Mutairi, S. R. Crosby, J. Darzi, J. R. Harding, R. A. Hughes, C. D. King, T. J. Simpson, R. W. Smith and C. L. Willis, Chem. Commun., 2001, 835 RSC.
- (a) J. S. Yadav, B. V. Subba Reddy, G. G. K. S. Narayana Kumar and M. G. Reddy, Tetrahedron Lett., 2007, 48, 4903 CrossRef CAS PubMed; (b) J. S. Yadav, B. V. Subba Reddy, S. Aravind, G. G. K. S. Narayana Kumar, C. Madhavi and A. C. Kunwar, Tetrahedron, 2008, 64(13), 3025–3031 CrossRef CAS PubMed.
- (a) U. C. Reddy, B. R. Raju, E. K. P. Kumar and A. K. Saikia, J. Org. Chem., 2008, 73, 1628 CrossRef CAS PubMed; (b) G. Sabitha, M. Bhikshapathi, S. Nayak, J. S. Yadav, R. Ravi and A. C. Kunwar, Tetrahedron Lett., 2008, 49, 5727 CrossRef CAS PubMed; (c) J. S. Yadav, B. V. S. Reddy, S. Aravind, G. G. K. S. N. Kumar, C. Madhavi and A. C. Kunwar, Tetrahedron, 2008, 64, 3025 CrossRef CAS PubMed.
- H. Banskota, Y. Tezuka, J. K. Prasain, K. Matsushige, I. Saiki and S. Kadota, J. Nat. Prod., 1998, 61, 896 CrossRef PubMed.
- T. C. McKee, R. W. Fuller, C. D. Covington, J. H. Cardellina, R. J. Gulakowski, B. L. Krepps, J. B. McMahon and M. R. Boyd, J. Nat. Prod., 1996, 59, 754 CrossRef CAS PubMed.
- (a) V. A. Ashwood, F. Cassidy, M. C. Coldwell, J. M. Evans, T. C. Hamilton, D. R. Howlett, D. M. Smith and G. Stemp, J. Med. Chem., 1990, 33, 2667 CrossRef CAS; (b) F. Cassidy, J. M. Evans, M. S. Hadley, A. H. Haladij, P. E. Leach and G. Stemp, J. Med. Chem., 1992, 35, 1623 CrossRef CAS.
- (a) K. S. Atwal, G. J. Grover, F. N. Ferrara, S. Z. Ahmed, P. G. Sleph, S. Dzwonczyk and D. E. Normandin, J. Med. Chem., 1995, 38, 1996 Search PubMed; (b) K. S. Atwal, G. J. Grover, S. Z. Ahmed, F. N. Ferrara, T. W. Harper, K. S. Kim, P. G. Sleph, S. Dzwonczyk, A. D. Russell, S. Moreland, J. R. McCullough and D. E. Normandin, J. Med. Chem., 1993, 36, 3971 CrossRef CAS.
- (a) P. Srinivasan, P. T. Perumal and S. Raja, Indian J. Chem., Sect. B: Org. Chem. Incl. Med. Chem., 2011, 50, 1083 Search PubMed; (b) B. V. Subba Reddy, K. Ramesh, A. V. Ganesh, G. G. K. S. Narayana Kumar, J. S. Yadav and R. Gree, Tetrahedron Lett., 2011, 52, 495 CrossRef PubMed; (c) G. Sabitha, M. Bhikshapathi, S. Nayak and J. S. Yadav, Synth. Commun., 2011, 41, 8 CrossRef CAS; (d) N. P. Selvam and P. T. Perumal, Can. J. Chem., 2009, 87, 698–705 CrossRef CAS.
- (a) J. S. Yadav, B. V. Subba Reddy, A. V. Ganesh and G. G. K. S. Narayan Kumar, Tetrahedron Lett., 2010, 51, 2963 CrossRef CAS PubMed; (b) G. Baishya, B. Sarmah and N. Hazarika, Synlett, 2013, 24, 1137 CrossRef CAS PubMed.
Footnote |
| † CCDC 968993. For crystallographic data in CIF or other electronic format see DOI: 10.1039/c4ra02124j |
| This journal is © The Royal Society of Chemistry 2014 |