Enhancing photocatalytic activity by tuning the ratio of hexagonal and orthorhombic phase Nb2O5 hollow fibers†
Abstract
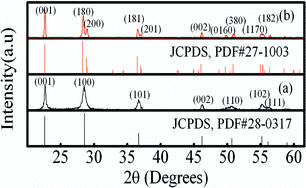
Cotton was used as template for the fabrication of a biomorphic Nb2O5 photocatalyst with hexagonal and orthorhombic structures. The as-prepared Nb2O5 was characterized through X-ray diffraction (XRD), scanning electron microscopy (SEM), specific surface area and UV-Vis diffused reflectance spectra measurements. The photocatalytic activity of the Nb2O5 fibers was evaluated through degradation of methylene blue (MB) in aqueous solution exposed to UV light. SEM results show that biomorphic Nb2O5 fibers are both hollow and have porous walls. The architectures allow a more accessible surface for the photocatalytic decomposition of dyes molecules. The photocatalytic activity of physically mixed fibers with 75% H-Nb2O5 and 25% O-Nb2O5 phases is superior to those of pure H- and O-Nb2O5 phases, and other samples with different H- and O-Nb2O5 ratios. Such superior photocatalytic activity is mainly due to an optimal balance between the adsorption of MB and the absorption of UV light. Moreover, the large size of the Nb2O5 hollow fibers allows them to be easily separated from the solution.

 Please wait while we load your content...
Please wait while we load your content...