High-temperature steam reforming of bio-oil derived light organics and methane to hydrogen-rich gas with trace CO via rational temperature control
Abstract
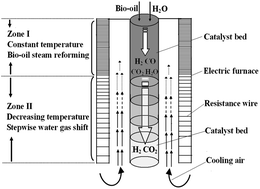
Steam reforming of methane or bio-oil is generally performed at high temperatures (>600 °C) to maintain the efficiency of the process. One main disadvantage of steam reforming at that high temperature is the formation of a large amount of CO due to the predomination of the reverse water gas shift reaction and the reformate gas with this level of CO cannot feed fuel cells. In this study a reactor with constant and decreasing temperature zones is developed to produce hydrogen-rich gas with trace CO from bio-oil derived light organics and methane. In the constant-temperature zone, the high temperature employed effectively promotes the reforming of organics and suppresses the generation of both complex organic by-products and coke. In the decreasing temperature zone, the CO produced in the constant-temperature zone is stepwisely and efficiently reduced to a ppm level using steam. In addition, the coke distribution along the catalyst bed varied a lot in the constant-temperature zone and the decreasing temperature zone, due to the different reaction network in the different temperature ranges. Via rational reaction temperature control, the efficient reforming of methane and the bio-oil derived light organics and the simultaneous elimination of CO is successfully achieved in one step in one reactor.

 Please wait while we load your content...
Please wait while we load your content...