Fluorenyl phenolphthalein groups containing a multi-block copolymer membrane for alkaline fuel cells†
Ajay K. Singhac,
Ravi P. Pandeyab and
Vinod K. Shahi*ab
aElectro-Membrane Processes Division, CSIR-Central Salt and Marine Chemicals Research Institute (CSIR-CSMCRI), Council of Scientific & Industrial Research (CSIR), Gijubhai Badheka Marg, Bhavnagar-364 002, Gujarat, India. E-mail: vkshahi@csmcri.org; vinodshahi1@yahoo.com; Fax: +91-0278-2566970
bAcademy of Scientific and Innovative Research, CSIR-Central Salt and Marine Chemicals Research Institute (CSIR-CSMCRI), Council of Scientific & Industrial Research (CSIR), Bhavnagar-364 002, Gujarat, India
cDepartment of Chemical Engineering, POSTECH (Pohang University of Science and Technology), Pohang, 790-784, Korea
First published on 24th April 2014
Abstract
An anion-conducting aromatic multi-block copolymer (PEs-AxBy) was synthesized by a block co-polycondensation reaction between fluorene and phenolphthalein containing oligomers. The quaternized multi-block copolymers (QPEs) resulted in ductile transparent membranes with well-ordered multi-block structures. In chloroform, a thin polymer film (PE-A22B24) with hexagonal well-ordered geometry was obtained. Meanwhile, in NMP/DMAc a dense thin film for QPE-A22B24 was obtained. As an additional attractive feature, the pore structure and pore-geometry of the membrane thin film may be controlled by the membrane casting medium and conditions. The presence of hydrophilic (biphenyl fluorene)–hydrophobic (connector)–hydrophilic (phenolphthalein) groups was responsible for phase separation, and interconnected ion conducting pathways. Among many synthesized anion exchange membranes (AEMs), QPE-A22B24 exhibited excellent stabilities, 0.95–2.24 meq g−1 ion exchange capacity and 95 mS cm−1 conductivity, at 80 °C. The reported AEM retained the conductivity after 1000 h of boiling treatment. QPE-A22B24 membranes were assessed as suitable candidates for alkaline fuel cell applications.
1. Introduction
Proton exchange membrane fuel cells (PEMFCs) have attracted extensive attention as clean and renewable energy sources.1 The commercialized perfluorosulfonic acid based polymer electrolyte membrane (PEM) (Nafion; DuPont), is state-of-the-art for PEMFCs because of high proton conductivity, thermal, chemical and mechanical stabilities.2 Requirement of precious metal (platinum) electro-catalysts, insufficient stability of membranes under fuel cell operating conditions, environmental incompatibility and high cost of perfluorinated membrane impede commercialization of PEMFCs.3Anion exchange membrane fuel cell (AEMFC) was proposed as an alternative,4 because of good oxygen reduction kinetics, transition metal (Co, Ni, etc.) electro-catalysts and relatively high fuel cell performance.5 In addition, AEMFCs offer fuel flexibility (e.g. methanol, ethanol, ethylene glycol, etc.), low over-potential for fuel oxidation and fuel crossover.6 Variety of anion exchange membranes (AEMs) based on organic–inorganic hybrid materials,2,7 aliphatic ethers,8 acrylate derivative,9 aromatic compounds,10 polyamides,11 polyacrylamide,12 poly aromatic ether,13 polyether amide,14 fluoro backbone,15 were reported.
Intensive research efforts were devoted to develop the AEMs with well defined hydrophilic–hydrophobic segments for improved IEC, conductivity, thermal stability, transparency, reflecting index, and low dielectric constant.16 We are reporting a multi-block copolymers containing hydrophilic (biphenyl fluorene)–hydrophobic (connector)–hydrophilic (phenolphthalein) blocks to achieve significant phase separation. Due to bulky aromatic structure (phenolphthalein and fluorenyl), breath figures were formed by water condensation employed as a physical micropattern,17 with good stabilities. Incorporation of phenolphthalein and fluorenyl blocks,18 and rigid bisphenol with pendant lactone are expected to provide reactive sites for grafting the functional groups.19
We designed well-ordered multi-block copolymers (PE-AxBy) containing phenolphthalein–fluorene oligomers. Three types of multi-block copolymers (PE-A12B18, PE-A18B20, and PE-A22B24) with varied block lengths were synthesized via coupling reactions. Controlled chloromethylation and ammination at specific position of fluorenyl and lactone group of the poly (aryl ethers) was achieved via Friedal–Crafts alkylation reaction.
2. Experimental section
2.1 Materials
4,4′-Diflurophenyl sulfone (FPS), 4,4′-difluorobenzophenone, 9,9′-bis(4-hydroxyphenyl)fluorene (BHF), phenolphthalein, chloromethyl methyl ether (CMME), trimethylamine (35 wt%) were purchased from Aldrich Chemicals and used as received. 1,1,2,2-Tetrachloroethane (TCE), N,N-dimethylacetamide (DMAc), methanol, acetone, chloroform, toluene, tetrahydrofuran, ethanol, K2CO3, NaOH, and ZnCl2 (anhydrous) were obtained from S.D. Fine chemicals, India, and used without further purification. Deionized (DI) water obtained from Milli-Q system was used for all experiments.2.2 Preparation of multi-block copolymer
A typical procedure for preparation of precursor multi-block copolymers (PEs-AxBy) (x and y represents the number of repeat units in the hydrophilic segments) has been described below.2.3 Chloromethylation of multi-block copolymer
For chloromethylation reaction, a 50 mL pressure glass bottle was charged with PE-A22B24 (1.0 g) and TCE (20 mL) with continuous stirring condition. After dissolution of copolymer (ambient temperature), ZnCl2 (0.1414 g) solution in THF (1.0 mL) and CMME (3.5 mL) were added. The reaction was carried out at 45 °C for 180 h to obtain yellow mixture. Obtained mixture was diluted with ca. 20 mL of TCE and poured drop wise into excess of methanol. The crude product was washed with deionized water and methanol several times. White powdered chloromethylated copolymer was obtained after filtration and drying at 80 °C under vacuum.2.4 Membrane preparation and quaternization
The chloromethylated copolymer CPE-A22B24 (1.000 g) was dissolved in TCE (25 mL) and solution was filtered with Whatman filter paper (0.45 μm). The filtrate was cast as thin film onto a flat glass plate and dried at 60 °C overnight to obtain transparent, tough membrane (60 ± 5 μm). Quaternization was achieved by aqueous trimethylamine solution (30 wt%) at room temperature (48 h). Obtained quaternized membrane was washed with water several times and immersed in NaOH solution (1 M) for 48 h to convert into alkaline form. The membrane was characterized using different instruments; water uptake, hydrolytic stability, ion exchange capacity, counter ion transport number, and membrane conductivity, described in ESI (Section S1–S6).†3. Results and discussion
3.1 Synthesis of precursor and multi-block copolymers
Series of precursors (poly(arylene ether sulphone ketone)) containing fluorenyl and phthalide groups were synthesized by nucleophilic substitution reaction (Scheme 1). The polymers were designated as PE-A12B18, -A18B20, -A22B24 (where A and B represent hydrophilic block, and numerical value represents the repeating unit). Their chemical structures were confirmed by 1H, 13C NMR, FT-IR, elemental analysis and molecular weight by GPC measurements (Fig. S1–S6, ESI† and Tables 1 & 2). Length of oligomers was determined by integral ratio between terminal shorten peak and their perspective polymerized proton peak. Length of fluorenyl group containing oligomer (A) was shorter than calculated feed amount. While phthalide group containing oligomer (B) showed less variation. Heating time (3–5 h) was kept less for both cases to avoid the unfavourable side chain reaction. Synthesized hydrophilic block and multiblock copolymer were soluble in organic solvents (chloroform, dichloroethane, 1,1,2,2-tetrachloroethane, DMAc, DMF, THF, and DMSO). Molecular weight of synthesized precursor and polymer was measured by GPC (Tables 1 & 2). Average molecular weight (Mn) of polymers was several times higher than the corresponding copolymer. Structure of multi-block copolymer was proposed by 1H & 13C NMR and FT-IR studies (Scheme 1). Good agreement between copolymer composition and oligomer feed ratio, confirmed controlled polymerizations.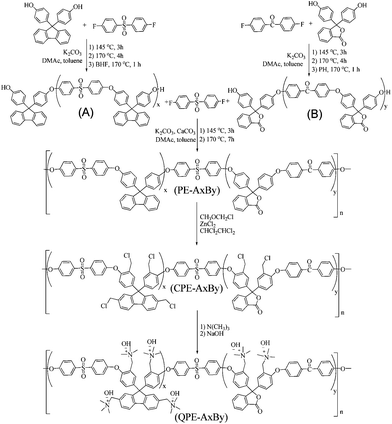 | ||
| Scheme 1 Synthesis of multi-block copoly(arylene ether)s containing fluorene and phenolphthalein groups. | ||
| Oligomer | Expected oligomer length | Obtained oligomer lengtha | Obtained oligomer lengthb | Mn | Mw | Mp | Mz | Mz+1 | Mn/Mw |
|---|---|---|---|---|---|---|---|---|---|
| a Estimated from the 1H NMR spectra.b Estimated from the GPC data. | |||||||||
| A12 | 18 | 12 | 12 | 6585 | 7265 | 7868 | 7929 | 8543 | 1.103 |
| A18 | 20 | 18 | 17 | 9607 | 9987 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 021 021 |
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 375 375 |
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 761 761 |
1.040 |
| A22 | 24 | 22 | 25 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 403 403 |
21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 283 283 |
20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 938 938 |
28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 396 396 |
35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 266 266 |
1.477 |
| B18 | 18 | 18 | 24 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 029 029 |
18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 065 065 |
18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 065 065 |
16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 796 796 |
32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 291 291 |
1.501 |
| B20 | 20 | 20 | 25 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 817 817 |
17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 840 840 |
16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 364 364 |
23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 670 670 |
29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 681 681 |
1.392 |
| B24 | 24 | 24 | 28 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 569 569 |
20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 379 379 |
19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 227 227 |
27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 224 224 |
34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 398 398 |
1.399 |
| Polymer | Expected A/B length | Obtained A/B length | Mn | Mw | Mw/Mn |
|---|---|---|---|---|---|
| PE-A12B18 | 18/18 | 12/18 | 61![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
77![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 978 978 |
1.270 |
| PE-A18B20 | 20/20 | 18/20 | 62![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
76![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 872 872 |
1.228 |
| PE-A22B24 | 24/24 | 22/24 | 77![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4000 4000 |
1.342 |
3.2 Synthesis and characterization of chloromethylated well-ordered multi-block copolymers (CPEs-AxBy)
Chloromethylation of multi-block copolymer was achieved by the method reported earlier16b (Scheme 1). Chemical structure and degree of chloromethylation (DC) for multi-block copolymer were estimated by elemental analysis, 1H & 13C NMR, and FT-IR spectra. 1H NMR spectra for chloromethylated polyether (CPE) (Fig. 1) showed two new peaks at 4.59 and 4.62 ppm assigned to methylene protons in –CH2Cl group (absent in precursor PE). Peaks between 6.8 and 7.8 ppm were altered (Fig. 1b) due to chloromethylation at 2- & 7-position of fluorenyl group and 2′-position of phthalimide group. | ||
| Fig. 1 1H NMR spectra for: (a) PE-A22B24; (b) CPE-A22B24; and (c) QPE-A22B24 (IEC = 1.8 meq g−1) membranes. | ||
DC values were calculated by integral ratio between peaks aroused at 4.59 and 4.63 ppm, for multi-block copolymer and increased with time (1H NMR spectra) (Fig. 2, and S7, ESI†). In 13C NMR, two new peaks at 45.1 and 46.9 ppm were generated due to methylene carbon (Fig. S8, ESI†), which indicate chloromethylation of phthalide groups. DC values are depicted in Table 3. At 45 °C, DC value was increased with reaction time (Fig. 2). Beyond 2.35 chloromethyl groups per repeated unit, polymer was gelated (insoluble in any organic solvent) due to high degree of cross-linking. Variation of peak was further confirmed by the FT-IR (Fig. S9, ESI†).
| Membrane | Reaction timea (h)/DC | IECb (meq g−1) | IECc (meq g−1) | Water uptake (%) | λX | t![[m with combining macron]](https://www.rsc.org/images/entities/char_006d_0304.gif) |
κmd (mS cm−1) | Φw | Φm | P/10−7 cm2 S−1 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 30% (MeOH–H2O) | 50% (MeOH–H2O) | ||||||||||
| a Reaction temperature: 45 °C; degree of chloromethylation calculated by 1H NMR.b IEC calculated by 1H NMR.c 45 °C: IEC calculated from titration methods.d κm values were measured at 60 °C in water.e λX represents number of absorbed water molecules per ammonium group.16b | |||||||||||
| QPE-A12B18 | 24/0.62 | 0.58 | 0.55 | 22.26 | 22.46 | 0.73 | 3.7 | — | — | — | — |
| 48/1.52 | 1.47 | 1.33 | 37.76 | 15.75 | 0.85 | 40 | — | — | — | — | |
| 180/2.12 | 2.05 | 1.95 | 59.92 | 17.05 | 0.90 | 73 | — | — | — | — | |
| QPE-A18B20 | 24/0.95 | 0.86 | 0.83 | 28.57 | 19.10 | 0.77 | 8.2 | — | — | — | — |
| 48/1.80 | 1.75 | 1.71 | 40.36 | 13.09 | 0.88 | 53 | — | — | — | — | |
| 180/2.25 | 2.16 | 2.10 | 66.03 | 17.45 | 0.93 | 79 | — | — | — | — | |
| QPE-A22B24 | 24/1.05 | 0.98 | 0.95 | 24.30 | 14.19 | 0.81 | 12 | 0.27 | 0.34 | 5.3 | 12.1 |
| 48/1.95 | 1.84 | 1.80 | 50.90 | 15.69 | 0.90 | 59 | 0.30 | 0.38 | 1.1 | 8.4 | |
| 180/2.35 | 2.29 | 2.24 | 82.46 | 20.42 | 0.94 | 91 | 0.35 | 0.40 | 0.7 | 3.2 | |
3.3 Membrane preparation and quaternization
The chloromethylated multi-block copolymers (CPEs) were dissolved in TCE for membrane casting (100 μm). Transparent membranes with ductile nature were dipped in trimethylamine solution (35 wt%) for 48 h at room temperature for quaternization (Fig. S10a, ESI†). Pale yellow coloured transparent quaternized membranes (QPEs) (Fig. S10b & c, ESI†) were equilibrated in NaOH (1 M) for 24 h at room temperature to convert into hydroxide form. QPEs membranes were less soluble in organic solvent (DMSO, CHCl3, NMP, DMAc, TCE etc.) and insoluble in acetone, THF, and methanol. The chemical structure of the QPEs were analysed by 1H, 13C NMR, and FT-ATR spectra (Fig. S11–S13, ESI†). 1H NMR spectrum for QPE-A22B24 (IEC: 1.80 meq g−1) showed two strong peaks at 2.93 and 3.01 ppm, assigned to methyl proton of quaternary ammonium groups (Fig. 1c). 1H NMR spectrum for QPE-A22B24 membrane (IEC: 2.24 meq g−1) was not recorded due to its low solubility in common organic solvents. Substitution of chloro- with ammonium groups was confirmed by magnetic field shifting from 4.62 ppm to 4.82 ppm.Integral ratios between two peaks (4.85 and 2.93–3.01 ppm) were quantitatively used to determine the IEC values (Table 3). IEC values obtained by classical titration were compared with those obtained from NMR spectra, and good agreement was found. Different carbon peaks (13C NMR spectra) are systematized for QPE membranes in Fig. S12 (ESI).† Disappearance of carbon peaks at 45.1 and 46.9 (CPEs), and appearance of additional peaks at 52.60, 53.00, 64.44, and 69.33 ppm (QPEs) confirmed quaternization. FT-ATR spectra also showed new absorption bands at 1685 and 3295 cm−1, due to quaternary ammonium groups (Fig. S13, ESI†).20
3.4 Surface morphology and dynamic radius
Surface morphology of polymer and membrane were studied by SEM and AFM analysis (Fig. 3). Polymer PE-A22B24 was dissolved in chloroform and an aliquot of 100 μL of polymer solution was cast onto the copper stub at 25 °C (90% humidity of casting chamber) and dried. SEM image for resultant membrane showed hexagonal well-ordered geometry (Fig. 3a), which was further confirmed with vertically aligned pore (1.62 μm) by AFM analysis (Fig. 3b). Pore size and arrangement were altered with decrease in humidity of casting chamber (Fig. 3a, c & d). This experiment was repeated six times and similar observations were recorded. Same morphology was also obtained in low boiling solvent (THF, CS2, DCM, Acetone etc.) due to formation of breath figures by water condensation.17a Highly concentrated (>10 mg mL−1) polymer solution was casted into thin film and dried under low humidity (<10%) at high temperature (>40 °C). No well-defined morphology was observed in the prepared membrane, which supported formation of breath figures. Under dissolution of polymer in NMP, DMAc, or TCE (high boiling solvent), dense membrane structure without hexagonal morphology or cracks and holes was obtained (Fig. 3e & f and S10d, (ESI)†). Thus, as attractive feature of prepared polymer, pore structure and pore-geometry may be controlled by membrane casting conditions and medium.EDX results of QPE-A22B24 membrane (Fig. S10e, (ESI)†) showed that membranes are completely metal free (impurity). Average dynamic radius of synthesized oligomer, multi-block copolymer, and chloromethylated copolymer was estimated with DLS analysis at constant solute mole fraction (2 × 10−4) in chloroform solvent (Fig. S14, ESI†). Average radius of polymer increased during copolymerization, but after chloromethylation size was reduced may be due to partial cross-linking of block copolymer.
3.5 IEC, transport number, water uptake and hydroxide ion conductivity of quaternized membranes
The ion-exchange capacity (IEC) provides information for density of ionisable quaternary ammonium groups in the membrane matrix, which are responsible for hydroxide conductivity. IEC value depends on degree of chloromethylation of multi-block copolymer (Table 3). IEC value depends on degree of chloromethylation of multi-block copolymer (Table 3). IEC value for QPE-A22B24 (2.24 meq g−1) is even higher than Nafion 117 membrane (0.92 meq g−1).7 Excess of water uptake led to the membrane swelling and thus deterioration in membrane conductivity and stability. Transport number for hydroxyl ions in the membrane matrix (t![[m with combining macron]](https://www.rsc.org/images/entities/char_006d_0304.gif) ) was estimated by membrane potential measurements using Teorell–Meyer–Sievers's (TMS) theory (Table 3).21 t
) was estimated by membrane potential measurements using Teorell–Meyer–Sievers's (TMS) theory (Table 3).21 t![[m with combining macron]](https://www.rsc.org/images/entities/char_006d_0304.gif) values varied between 0.73 and 0.94, and increased with degree of chloromethylation.
values varied between 0.73 and 0.94, and increased with degree of chloromethylation.
Presence of water is necessary in the membrane matrix for hydroxide conduction. But, water uptake for QPEs membrane increased with IEC value (Table 3). QPE-A22B24 (IEC: 2.24 meq g−1) membrane showed 82.46 wt% water uptake at 30 °C, which is favourable for fuel cell application. In general, membrane contains free (absorbed by ionic groups and hydrophilic domains) and bound water in the matrix. Number of water molecules absorbed per ammonium group (λ) was estimated by IEC values (Table 3). Relatively high λ values for QPE-A12B18 (IEC: 0.55 meq g−1) membrane may be attributed to relatively large hydrophilic polymer chain responsible for more water absorption and therefore phase separation. Membrane conductivity for QPE membrane under 100% humidity conditions increased with temperature and IEC (Fig. 4). Membrane conductivity increased with IEC value. As a reference, QPE-A22B24 membrane (IEC: 2.24 meq g−1) showed 95 mS cm−1 conductivity at 80 °C. Interestingly, membrane conductivity also increased with molecular weight of polymer, due to polymer entanglement and high water uptake. Activation energy (Ea) was estimated from Arrhenius plot by following equation: (Fig. S15, ESI†)
| Ea = −b × R | (1) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) km vs. 1000/T (K−1) plots) and R is the gas constant (8.314 J−1 K−1 mol−1). The activation energy values (8–16 kJ mol−1) were independent on IEC and increased with molecular weight. The apparent activation energy of multi-block QPE membranes was comparable or somewhat lower than those of reported AEMs (9.92–23.03 kJ mol−1).16a
km vs. 1000/T (K−1) plots) and R is the gas constant (8.314 J−1 K−1 mol−1). The activation energy values (8–16 kJ mol−1) were independent on IEC and increased with molecular weight. The apparent activation energy of multi-block QPE membranes was comparable or somewhat lower than those of reported AEMs (9.92–23.03 kJ mol−1).16a
For QPE-A22B24 membranes, methanol permeability value was measured by method reported in Section S7 (ESI)† and included in (Table 3). Across prepared membranes, methanol permeability values increased with methanol concentration. Methanol permeability of QPE-A22B24 membrane (IEC: 2.24 meq g−1) is 0.7 × 10−7 cm2 s−1 at 30% MeOH, and 3.2 × 10−7 cm2 s−1 at room temperature. Compared to Nafion® membrane (13.2 × 10−7 cm2 s−1 at room temperature),2 QPE-A22B24 membrane (IEC: 2.24 meq g−1) has lowered methanol permeability. Lower methanol permeability values indicates that the methanol crossover rate for QPE-A22B24 membrane (IEC: 2.24 meq g−1) membrane will be potentially lower than that of Nafion® membrane, which may improve fuel cell performance.
Some salient features (IEC, and membrane conductivity) of reported multi-block copolymer AEMs in the literature are compared with prepared QPE-A22B24 membrane, in Table 4, under similar experimental conditions.16a,22–24 These properties of optimized QPE-A22B24 membrane are superior than other reported multi-block copolymer AEMs.
3.6 Stability, solvent uptake, and solubility studies of QPE membranes
Thermal stabilities of synthesized oligomer, polymer, CPEs and QPEs membranes were analysed by thermogravimetrical analysis (TGA) (Fig. S16, ESI†). All thermo-grams showed two-steps weight loss. First step weight loss (∼1%) was observed at 50–200 °C due to evaporation of absorbed/bound water. Second step weight loss (∼30%) between 465 and 700 °C was attributed to the polymer degradation. For CPE-A22B24 and QPE-A22B24 membranes, degradation peaks between 200 and 400 °C were assigned to the degradation of side chain (chloromethylated or amminated). In DSC studies under N2 environment between 30 and 300 °C, first endothermic transition temperature for all monomers and polymers (dry oligomer, polymer, CPE and QPE membranes) was lower than 100 °C, and area of endothermic regions increased with the step-wise progress of polymerization, and thus reduction in membrane crystallinity (Fig. S17, ESI†). The storage modulus (E′), loss modulus (E′′) and tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ for QPE-A22B24 (IEC: 2.24 meq g−1) membrane were determined by DMA at 10 Hz frequency between 30 and 410 °C (Fig. S18 (ESI)†). QPE-A22B24 membrane (IEC: 2.24 meq g−1) showed 0.83 GPa initial storage modulus, while 0.063 GPa loss modulus and 351 °C glass transition temperature was observed.
δ for QPE-A22B24 (IEC: 2.24 meq g−1) membrane were determined by DMA at 10 Hz frequency between 30 and 410 °C (Fig. S18 (ESI)†). QPE-A22B24 membrane (IEC: 2.24 meq g−1) showed 0.83 GPa initial storage modulus, while 0.063 GPa loss modulus and 351 °C glass transition temperature was observed.
The presence of water molecules in the membrane matrix facilitates the dissociation of functional groups and hydroxyl group transport. However, excessive water uptake deteriorated membrane mechanical properties and dimensional stability. For QPE-A22B24 membranes, change in volume fraction in water (Φw), and change in volume fraction in methanol (Φm), (Table 3) were estimated by method reported earlier.25 Water absorption occurred due to presence of hydrophilic phenolphthalein and quaternary ammonium groups. Methanol–water uptake was little high as compared to water uptake. Volume change by swelling in equilibration with water or methanol confirmed dimensional stability of prepared QPE-A22B24 membrane for fuel cell application.
Durability of QPE-A22B24 (IEC = 2.24 meq g−1) membrane was also tested in hot degassed deionised water (80 °C) for 1000 h (Fig. 5). Membrane conductivity was reduced up to 10 h, and afterwards it attained constant value. In similar fashion, after 1000 h, about 15% reduction in membrane IEC was observed. Thus reported anion conducting membranes are quite good durable and suitable for alkaline fuel cell application. Synthesized membrane QPE-A22B24, conductivity and IEC properties was compared with previous report showed that synthesized membrane had excellent AEM properties.
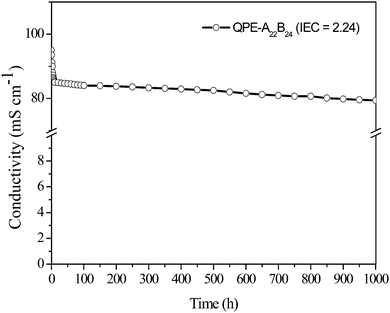 | ||
| Fig. 5 Time course of hydroxide ion conductivity for QPE-A22B24 (IEC = 2.24 meq g−1) membrane in water at 80 °C. | ||
4. Conclusions
A series of multi-block AEMs with high molecular weights containing phenolphthalein and fluorenyl groups were synthesized. Structure of the designed AEM was confirmed by different spectroscopic techniques. By intelligent optimization of reaction conditions and multi-block copolymers precursor, chloromethylation at specific positions for both blocks, was successfully achieved. Herein, we are reporting multi-block AEMs, in which both hydrophilic blocks were joined by a hydrophobic moiety, to achieve good hydrophobic–hydrophilic balance and hydroxide conduction. Membrane properties were highly dependent on DC/QC, and QPE-A22B24 (IEC = 2.24 meq g−1) membrane showed 2.35 DC value. It seems, nearly complete chloromethylation was achieved on fluorenyl group, due to high electron density. Partial chloromethylation on phenolphthalein group was assigned due to relatively low electron density. Conductivity, IEC, stabilities and durability assessment of QPE-A22B24 (IEC = 2.24 meq g−1) membrane revealed it a potential candidate for alkaline fuel cell application.These membranes were architected by introducing fluorenyl and phthalide groups and showed high phase separation, water content, stabilities and other desired properties. Concept of utilizing multi-block AEM based on high molecular weight aromatic polymers, containing hydrophilic blocks joined together by hydrophobic moiety, is verified for improving hydroxide conductivity without sacrificing stabilities and other desired properties for alkaline fuel cell application.
Acknowledgements
One of the authors (Ravi P. Pandey) is thankful to University Grand commission (UGC), New Delhi, for providing Senior Research Fellowship (SRF). Financial assistance of Ministry of New and Renewable Energy, New Delhi, Gov. of India, (project no. 102/79/2010-NT) is acknowledged. Instrumental support received from Analytical Science Division, CSIR-CSMCRI, is also gratefully acknowledged.References
- (a) K. Matsumoto, T. Fujigaya, H. Yanagi and N. Nakashima, Adv. Funct. Mater., 2011, 21, 1089 CrossRef CAS; (b) R. Akiyama, D. Hirayama, M. Saito, J. Miyake, M. Watanabe and K. Miyatake, RSC Adv., 2013, 3, 20202 RSC.
- T. Chakrabarty, A. K. Singh and V. K. Shahi, RSC Adv., 2012, 2, 1949 RSC.
- (a) E. Antolini and E. R. Gonzalez, J. Power Sources, 2010, 195, 3431 CrossRef CAS PubMed; (b) Z. Zhao, J. Wang, S. Li and S. Zhang, J. Power Sources, 2011, 196, 4445 CrossRef CAS PubMed.
- (a) H. Zhang and P. K. Shen, Chem. Rev., 2012, 112, 2780 CrossRef CAS PubMed; (b) X. Li, Y. Yu, Q. Liu and Y. Meng, ACS Appl. Mater. Interfaces, 2012, 4, 3627 CrossRef CAS PubMed.
- (a) Y. Zha, M. L. Disabb-Miller, Z. D. Johnson, M. A. Hickner and G. N. Tew, J. Am. Chem. Soc., 2012, 134, 4493 CrossRef CAS PubMed; (b) N. Li, Q. Zhang, C. Wang, Y. M. Lee and M. D. Guiver, Macromolecules, 2012, 45, 2411–2419 CrossRef CAS.
- (a) G. Couture, A. Alaaeddine, F. Boschet and B. Ameduri, Prog. Polym. Sci., 2011, 36, 1521 CrossRef CAS PubMed; (b) N. J. Robertson, H. A. Kostalik, T. J. Clark, P. F. Mutolo, H. D. Abruña and G. W. Coates, J. Am. Chem. Soc., 2010, 132, 3400 CrossRef CAS PubMed.
- B. P. Tripathi, M. Kumar and V. K. Shahi, J. Membr. Sci., 2010, 360, 90 CrossRef CAS PubMed.
- D. Stoica, F. Alloin, S. Marais, D. Langevin, C. Chappey and P. Judeinstein, J. Phys. Chem. B, 2008, 112, 12338 CrossRef CAS PubMed.
- Y. Luo, J. Guo, C. Wang and D. Chu, J. Power Sources, 2010, 195, 3765 CrossRef CAS PubMed.
- Q. H. Zeng, Q. L. Liu, I. Broadwell, A. M. Zhu, Y. Xiong and X. P. Tu, J. Membr. Sci., 2010, 349, 237 CrossRef CAS PubMed.
- Y. Pérez-Padilla, M. A. Smit and M. J. Aguilar-Vega, Ind. Eng. Chem. Res., 2011, 50, 9617 CrossRef.
- T. Nonaka and K. Fujita, J. Membr. Sci., 1998, 144, 187 CrossRef CAS.
- R. Bai, Q. Li, L. Liu, Q. Miao and B. Jin, Chem. Commun., 2014, 50, 2791–2793 RSC.
- G. Wang, Y. Weng, D. Chu, D. Xie and R. Chen, J. Membr. Sci., 2009, 326, 4 CrossRef CAS PubMed.
- K. Matsui, E. Tobita, K. Sugimoto, K. Kondo, T. Seita and A. Akimoto, J. Appl. Polym. Sci., 1986, 32, 4137 CrossRef CAS.
- (a) K. Miyatake, B. Bae and M. Watanabe, Polym. Chem., 2011, 2, 1919 RSC; (b) M. Tanaka, K. Fukasawa, E. Nishino, S. Yamaguchi, K. Yamada, H. Tanaka, B. Bae, K. Miyatake and M. Watanabe, J. Am. Chem. Soc., 2011, 133, 10646 CrossRef CAS PubMed.
- (a) A. Muñoz-Bonilla, E. Ibarboure, E. Papon and J. Rodriguez-Hernandez, Langmuir, 2009, 25, 6493 CrossRef PubMed; (b) P. Escalé, L. Rubatat, L. Billon and M. Save, Eur. Polym. J., 2012, 48, 1001 CrossRef PubMed.
- (a) R. Guo, O. Lane, D. VanHouten and J. E. McGrath, Ind. Eng. Chem. Res., 2010, 49, 12125 CrossRef CAS; (b) A. Gugliuzza, M. C. Aceto, F. Macedonio and E. Drioli, J. Phys. Chem. B, 2008, 112, 10483 CrossRef CAS PubMed.
- Y. Xiong, Q. L. Liu and Q. H. Zeng, J. Power Sources, 2009, 193, 541 CrossRef CAS PubMed.
- A. K. Singh, S. Prakash, V. Kulshrestha and V. K. Shahi, ACS Appl. Mater. Interfaces, 2012, 4, 1683 CAS.
- E.-Y. Choi, H. Strathmann, J.-M. Park and S.-H. Moon, J. Membr. Sci., 2006, 268, 165 CrossRef CAS PubMed.
- J. Wang, S. Li and S. Zhang, Macromolecules, 2010, 43, 3890 CrossRef CAS.
- L. Li and Y. Wang, J. Membr. Sci., 2005, 262, 1 CrossRef CAS PubMed.
- G. Wang, Y. Weng, D. Chu, R. Chen and D. Xie, J. Membr. Sci., 2009, 332, 63 CrossRef CAS PubMed.
- R. P. Pandey and V. K. Shahi, J. Membr. Sci., 2013, 444, 116 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: NMR data, FTIR, ATR spectra, DLS, TGA, DSC, DMA profile, photographs of the membrane, SEM image, detailed about used instrument, water uptake, hydrolytic stability, ion exchange capacity, counter ion transport number, and membrane conductivity are available in the online version of this article. See DOI: 10.1039/c4ra01999g |
| This journal is © The Royal Society of Chemistry 2014 |



