From graphite oxide to nitrogen and sulfur co-doped few-layered graphene by a green reduction route via Chinese medicinal herbs
Abstract
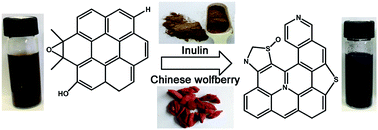
In this work, we developed a green and facile approach to prepare graphene by the reduction of graphite oxide (GO) using two Chinese medicinal herbs: inulin and Chinese wolfberry. The reduced products were systematically characterized by UV-visible absorption spectroscopy, X-ray diffraction, X-ray photoelectron spectroscopy, Raman spectroscopy, Fourier transform infrared spectroscopy, scanning electron microscopy and transmission electron microscopy. These results provided convincing evidence of the removal of oxygen-containing groups from GO to form few-layered graphene after the reduction reaction. Simultaneously, nitrogen and sulfur co-doping were achieved under a relatively low temperature (90 °C). The reduction mechanism of GO and N, S bonding configurations in graphene were also proposed.

 Please wait while we load your content...
Please wait while we load your content...