Local co-delivery and release of antimicrobial peptide and RGD using porous TiO2†
Junjian Chenabc,
Lin Wangabc,
Lin Shiabc,
Li Ren*abc and
Yingjun Wang*abc
aNational Engineering Research Center for Tissue Restoration and Reconstruction, South China University of Technology, Guangzhou 510006, China. E-mail: imwangyj@163.com; psliren@scut.edu.cn
bSchool of Materials Science and Engineering, South China University of Technology, Guangzhou 510641, China
cGuangdong Province Key Laboratory of Biomedical Engineering, South China University of Technology, Guangzhou 510006, China
First published on 23rd May 2014
Abstract
We demonstrated for the first time the use of two kinds of porous TiO2 films to co-deliver peptide HHC36 (KRWWKWWRR) and RGD. The film co-delivering these two peptides exhibited excellent antimicrobial activity against S. aureus and E. coli and low cytotoxicity to rat bone mesenchymal stem cells (rBMSCs).
1. Introduction
Porous TiO2 prepared by anodic oxidation is one of the potential biomaterials for hard tissue implants.1,2 As reported previously, it shows excellent biocompatibility and bioactivity in vitro and in vivo.3 However, for hard tissue implants, especially the titanium-matrix implants, peri-implant infections in early stage are common in implanting operations, which could lead to the failure of the surgery, patient disability, and even death.4 Many methods have been used to improve the antimicrobial activity of the implants such as UV treatment,5 overlaying an antimicrobial polymer on the implant film,6 incorporation with silver nanoparticles7 or local delivery of various antibiotics.8Among these methods, for titanium-matrix implants, antimicrobial peptides (AMPs), especially the short antimicrobial peptides,9,10 are being screened by researchers. Although they are formed by lesser amino acids, the short antimicrobial peptides have improved antimicrobial activity, broad-spectrum activity, low susceptibility for developing bacterial resistance and short contact time to induce killing.9 Moreover, after simple physical absorption, these peptides could be loaded and released from the porous TiO2 film to show excellent antimicrobial activity.11 However, in order to ensure the long actuation duration, excessive AMPs were often loaded on the porous TiO2 film.12 The large amount of AMPs could evidently lead to cytotoxicity and this would limit its further application in clinic.12,13
In this paper, in order to resolve the cytotoxicity of this controlled-release system, we use porous TiO2 film as the substrate to co-deliver HHC36 (KRWWKWWRR) and another kind of peptide, RGD, at the same time.14 The RGD peptide mainly exists in the extracellular matrix. It could specifically combine with 11 species of integrin and promote the conglutination between matrix and implants, which could apparently improve the biocompatibility of the implants.15,16 We first prepared two kinds of porous TiO2 on pure titanium in organic or inorganic solutions.17,18 Then, we used the film to load these two kinds of peptides at different molar ratios, and characterized the co-release of the peptides from the film. The antimicrobial activity of the film against S. aureus and E. coli was tested in vitro, and the biocompatibility of the film was tested with rat bone mesenchymal stem cells (rBMSCs).
2. Experimental
2.1 Materials
Pure titanium foil (10 × 10 × 0.3 mm3, 99.8% purity) was purchased from Chenhui Metal Materials Ltd. (Baoji, China). The peptides HHC36 and RGD were purchased from GL Bio (GL Biochem (Shanghai) Ltd.). The porous TiO2-related reagents were purchased from Guangzhou Chemical Factory Co. Ltd. (Guangdong, China). The bacteria and cell-related reagents were purchased from Sigma-Aldrich (USA).2.2 Anodic oxidation
The titanium foils were treated with 3 vol% of HF and 5 vol% of HNO3. Then, they were washed with acetone, ethanol and distilled water for 10 min and used as the anode, while the platinum foil was used as the cathode. This system was immersed into electrolyte solution containing 0.27 M NH4F in 75% glycerol (organic electrolyte) at 30 V for 6 h or immersed into electrolyte solution containing 1 M (NH4)2SO4 and 0.5 wt% NH4F (inorganic electrolyte) at 30 V for 0.5 h. After anodization, the foils were washed with ethanol for 15 min by ultrasonication, and then the foils were annealed from room temperature to 500 °C at the rate of 5 °C min−1, held for 3 h, and cooled down in the furnace. The foils treated in organic and inorganic electrolytes were denoted as Org and Inorg, respectively.2.3 Loading peptides onto the porous TiO2
The RGD peptide was added into a solution of AMP (HHC36, 1 mM in ethanol) at various molar concentrations (0, 1, 2 and 3 mM). Then, 50 μL of the solution was added onto the porous TiO2 film, and the film was dried under vacuum desiccator at room temperature for 30 min, and this process was repeated five times. After that, the film was washed with PBS three times. The Org films treated with the solution containing AMP and RGD at different molar concentrations were abbreviated as Org-AMP, Org-AMP-1RGD, Org-AMP-2RGD and Org-AMP-3RGD, while the Inorg films treated with the solution containing AMP and RGD at different molar concentrations were abbreviated as Inorg-AMP, Inorg-AMP-1RGD, Inorg-AMP-2RGD and Inorg-AMP-3RGD. The films treated with the solution containing only 1 mM RGD were abbreviated as Org-RGD and Inorg-RGD.2.4 The characterization of the film
We used an ELISA plate reader (Varioskan Flash 3001, Thermo, Finland) to quantify the release rate of AMP, and the BCA kit to calculate the release rate of RGD. Staphylococcus aureus (S. aureus, strain ATCC 29213) and Escherichia coli (E. coli, strain ATCC 15224) were used to test the antimicrobial activity of the film, whereas rBMSCs were used to test the biocompatibility of the film. The details of the experiments are described in the ESI.†3. Results and discussion
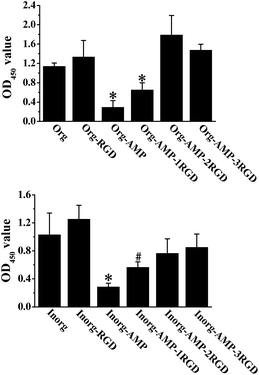
The SEM images of the films, which are shown in Fig. 1S,† showed that the average pore size of the Inorg film was about 125 nm, while that of Org film was about 70 nm. In addition, the porous structure on the Inorg film was more regular. The XRD results in Fig. 2S† showed that after annealing, the phase transformed from amorphous to anatase. These results demonstrated that due to the high specific surface area and cell-favourite crystal, our films could be beneficial to be used as hard tissue implants to load with peptides, as shown in other references.2,19We then used the CCK-8 assay to test the biocompatibility of different films to rBMSCs. The results shown in Fig. 1 show that compared to the control (Org and Inorg), the films loaded with only AMP (Org-AMP and Inorg-AMP) exhibited obvious cytotoxicity, which could kill about 74.48% and 72.26% of cells on them, respectively; moreover, the RGD on the film could improve the biocompatibility. Org-RGD and Inorg-RGD films, which were the films loaded with only RGD, exhibited better biocompatibility compared with Org or Inorg, and the OD values increased about 17.39% and 21.50%, respectively. In addition, for the co-delivery system, the cells on Org-AMP-1RGD and Inorg-AMP-1RGD increased by about 1.24 and 0.98 times compared to Org-AMP and Inorg-AMP, respectively. With the increase of RGD, the biocompatibility of the films improved. Compared to Org-AMP and Inorg-AMP, the cells on Org-AMP-2RGD and Inorg-AMP-2RGD increased by 5.16 and 1.67 times, respectively, while the cells on Org-AMP-3RGD and Inorg-AMP-3RGD increased by 4.08 and 1.97 times, respectively.
As the film had excellent biocompatibility when the molar ratios of AMP and RGD were 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 or 1
2 or 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 (shown in Fig. 1), we chose Org-AMP-2RGD and Inorg-AMP-2RGD for the next experiment. In order to illustrate the effect of the peptides more clearly, we stained the cells on different films with FITC dye after culturing for 4 and 24 hours, and the fluorescent images are shown in Fig. 3S and Fig. 4S.† The figure shows that at 4 hours, Org-AMP-2RGD showed biocompatibility similar to Org, which was better than Org-AMP but poorer than Org-RGD. This might be caused by the fact that, although the AMP in both Org-AMP and Org-AMP-2RGD exhibited cytotoxicity, the RGD could improve the adhesion of cells early on. After 24 h, Org-AMP-2RGD had biocompatibility similar to Org-RGD, which was a little better than Org; moreover, the cells on Org-AMP were less evident than others. This result corresponded to the CCK-8 results shown in Fig. 1, and it demonstrated that the RGD peptide in the co-delivery system could also improve the proliferation of the cells.
3 (shown in Fig. 1), we chose Org-AMP-2RGD and Inorg-AMP-2RGD for the next experiment. In order to illustrate the effect of the peptides more clearly, we stained the cells on different films with FITC dye after culturing for 4 and 24 hours, and the fluorescent images are shown in Fig. 3S and Fig. 4S.† The figure shows that at 4 hours, Org-AMP-2RGD showed biocompatibility similar to Org, which was better than Org-AMP but poorer than Org-RGD. This might be caused by the fact that, although the AMP in both Org-AMP and Org-AMP-2RGD exhibited cytotoxicity, the RGD could improve the adhesion of cells early on. After 24 h, Org-AMP-2RGD had biocompatibility similar to Org-RGD, which was a little better than Org; moreover, the cells on Org-AMP were less evident than others. This result corresponded to the CCK-8 results shown in Fig. 1, and it demonstrated that the RGD peptide in the co-delivery system could also improve the proliferation of the cells.
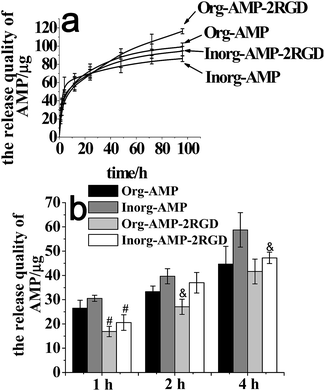
We then detected the release of the peptides from the films. The release curve of AMP from the films (Org-AMP, Org-AMP-2RGD, Inorg-AMP and Inorg-AMP-2RGD) was detected by ELISA and the results are shown in Fig. 2(a). The burst release of AMP from films was detected in the first 4 h in Fig. 2(a). About 45.03%, 35.77%, 68.12% and 49.98% of the AMP was released from Org, Org-AMP-2RGD, Inorg and Inorg-AMP-2RGD during this stage, respectively. However, this result was better compared to others,9 which would release about 81.7% AMPs during the burst stage in the first 4 h. Fig. 2(b) shows the release of AMP from indicated films in the first 4 h. Interestingly, it showed that the incorporation of RGD could improve the burst release of AMP at an early stage (especially at 1 h). That could be due to the possible interaction between the two peptides, which could alter the diffusion kinetics between them.20 The release curves of RGD for different films detected by BCA kit are shown in Fig. 5S,† which also shows the burst release in the first 4 h.
In order to determine the effect of the peptides released from the films on the biocompatibility, we tested the rBMSCs' viability in the medium containing the AMP or RGD at certain concentrations, and the results are shown in Fig. 6S and 7S.† The release curves in Fig. 2(a) showed that about 50–80 μg mL−1 of AMP released into the medium in the first 24 h. At these concentrations, the AMPs showed evident cytotoxicity to the rBMSCs (as shown in Fig. 6S†), which illustrated that the cytotoxicity of the films containing AMP could be caused by the high concentrations of the peptide. Fig. 5S† showed that about 80–140 μg mL−1 RGD was released in the first 24 h. Interestingly, the rBMSCs' viability in the medium containing the RGD at these concentrations did not show an evident difference (Fig. 7S†). This illustrated that the better biocompatibility of the co-delivery film might be caused by the RGD left on the surface.
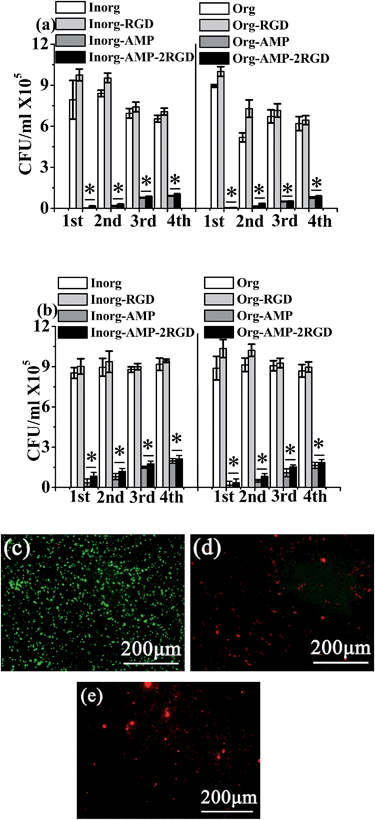
The antimicrobial activity of different films was tested with S. aureus and E. coli, and the results are shown in Fig. 3. The results demonstrated that the Org-AMP and Inorg-AMP films showed excellent antimicrobial activity against S. aureus and E. coli. Moreover, after being loaded with RGD, the antimicrobial activity of the films (Org- AMP-2RGD and Inorg-AMP-2RGD), which could also kill almost 100% of bacteria in 30 min, did not decrease. In addition, the Org-RGD and the Inorg-RGD films did not evidently kill the bacteria, which demonstrated that the RGD peptide had no antimicrobial activity. The consecutive killing assays, which indicates reusing the samples after the last antimicrobial test, also demonstrated that the antimicrobial activity of the films could maintain stability after four cycles; moreover, after the fourth round, compared to the control (Org and Inorg), the Org-AMP, Inorg-AMP, Org-AMP-2RGD and Inorg-AMP-2RGD could also kill about 87.39%, 86.08%, 85.75% and 84.30% of S. aureus, and 81.19%, 78.58%, 78.69% and 76.95% of E. coli, respectively. The live/dead assay images shown in Fig. 3(c)–(e) and 8S† also showed that these antimicrobial films exhibited excellent antimicrobial activity, while the live bacteria exhibited green fluorescence and the dead bacteria exhibited red fluorescence.
4. Conclusions
We have successfully used RGD to improve the biocompatibility of two kinds of porous TiO2 films loaded with AMP. When the molar ratio of AMP and RGD is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, there is significant increase in the biocompatibility of the film. Moreover, these films show excellent antimicrobial activity against S. aureus and E. coli even after four cycles. Therefore, this co-delivery system could be a potential solution for early stage peri-implant infection.
2, there is significant increase in the biocompatibility of the film. Moreover, these films show excellent antimicrobial activity against S. aureus and E. coli even after four cycles. Therefore, this co-delivery system could be a potential solution for early stage peri-implant infection.
Acknowledgements
The authors gratefully acknowledge the financial support of National Natural Science Foundation of China (Grant 51232002), the Basic Research Project of China (2012CB619100), the National Natural Science Foundation of China (Grant 51302088), the China Postdoctoral Science Foundation (Grant 2013M531844) and the Fundamental Research Funds for the Central Universities (Grant 2013ZB0001).Notes and references
- P. Chennell, E. Feschet-Chassot, T. Devers, K. O. Awitor, S. Descamps and V. Sautou, Int. J. Pharm., 2013, 455, 298–305 CrossRef CAS PubMed.
- R. Narayanan, T.-Y. Kwon and K.-H. Kim, Mater. Chem. Phys., 2009, 117, 460–464 CrossRef CAS PubMed.
- X. P. Fan, B. Feng, Z. Y. Liu, J. Tan, W. Zhi, X. Lu, J. X. Wang and J. Weng, J. Biomed. Mater. Res., Part A, 2012, 100A, 3422–3427 CrossRef CAS PubMed.
- R. O. Darouiche, N. Engl. J. Med., 2004, 350, 1422–1429 CrossRef CAS PubMed.
- A. Kubacka, M. J. Munoz-Batista, M. Ferrer and M. Fernandez-Garcia, Appl. Catal., B, 2013, 140, 680–690 CrossRef PubMed.
- L. Wang, U. J. Erasquin, M. R. Zhao, L. Ren, M. Y. Zhang, G. J. Cheng, Y. J. Wang and C. Z. Cai, ACS Appl. Mater. Interfaces, 2011, 3, 2885–2894 CAS.
- V. Sambhy, M. M. MacBride, B. R. Peterson and A. Sen, J. Am. Chem. Soc., 2006, 128, 9798–9808 CrossRef CAS PubMed.
- S. R. Giddens and D. C. Bean, Int. J. Antimicrob. Agents, 2007, 29, 93–97 CrossRef CAS PubMed.
- M. H. Ma, M. Kazemzadeh-Narbat, Y. Hui, S. S. Lu, C. F. Ding, D. D. Y. Chen, R. E. W. Hancock and R. Z. Wang, J. Biomed. Mater. Res., Part A, 2012, 100A, 278–285 CrossRef CAS PubMed.
- M. D. Seo, H. S. Won, J. H. Kim, T. Mishig-Ochir and B. J. Lee, Molecules, 2012, 17, 12276–12286 CrossRef CAS PubMed.
- M. Zasloff, Nature, 2002, 415, 389–395 CrossRef CAS PubMed.
- C. Wang, L.-L. Tian, S. Li, H.-B. Li, Y. Zhou, H. Wang, Q.-Z. Yang, L.-J. Ma and D.-J. Shang, PLoS One, 2013, 8, e60462 CAS.
- Y. Li, C. M. Santos, A. Kumar, M. R. Zhao, A. I. Lopez, G. T. Qin, A. M. McDermott and C. Z. Cai, Chem.–Eur. J., 2011, 17, 2656–2665 CrossRef CAS PubMed.
- F. Schmidt-Stein, R. Hahn, J.-F. Gnichwitz, Y. Y. Song, N. K. Shrestha, A. Hirsch and P. Schmuki, Electrochem. Commun., 2009, 11, 2077–2080 CrossRef CAS PubMed.
- U. Hersel, C. Dahmen and H. Kessler, Biomaterials, 2003, 24, 4385–4415 CrossRef CAS.
- E. Ruoslahti and M. D. Pierschbacher, Science, 1987, 238, 491–497 CAS.
- R. Sanchez-Tovar, K. Lee, J. Garcia-Anton and P. Schmuki, Electrochem. Commun., 2013, 26, 1–4 CrossRef CAS PubMed.
- W. T. Peng, Z. M. Qiao, Q. Zhang, X. D. Cao, X. F. Chen, H. Dong, J. W. Liao and C. Y. Ning, J. Mater. Chem. B, 2013, 1, 3506–3512 RSC.
- N. Wang, H. Li, W. Lue, J. Li, J. Wang, Z. Zhang and Y. Liu, Biomaterials, 2011, 32, 6900–6911 CrossRef CAS PubMed.
- H. Layman, M. Sacasa, A. E. Murphy, A. M. Murphy, S. M. Pham and F. M. Andreopoulos, Acta Biomater., 2009, 5, 230–239 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Details of antimicrobial assay and biocompatibility assay, as well as the SEM image, XRD pattern of the porous TiO2, the release curve of RGD, the fluorescent image of bacterial live/dead assay. See DOI: 10.1039/cc4ra01983k |
| This journal is © The Royal Society of Chemistry 2014 |