Effect of metal ions on the morphology of silver nanocrystals†
Gao Minga,
Ma Lirana,
Gao Yuana,
Guo Dana,
Wang Dingshengb and
Luo Jianbin*a
aState Key Laboratory of Tribology, Beijing 100084, China. E-mail: luojb@mail.tsinghua.eud.cn
bDepartment of Chemistry, Tsinghua University, Beijing 100084, China
First published on 6th May 2014
Abstract
We developed a facile synthesis based on benzyl alcohol for Ag NCs. The effect of metal ions on the formation of silver nanocrystals was investigated. Ag nanoplates were obtained with the assistance of Al3+ ions or Fe3+ ions, while other metal ions had no influence and nanoparticles were produced instead.
Shape control of metallic nanostructures has been the focus of intensive research for decades due to their widespread applications in optics, electronics, magnetics, catalysis, and the intrinsic properties of metal nanocrystals have been demonstrated to depend on their exact size and morphology.1–5 Compared with other noble metal nanostructures, silver nanoparticles are of especial note because of their unique and tunable surface-plasmonic features, which promise a superior performance in many fields.
Over the past decades, different shapes of silver nanoparticles have been obtained via various syntheses, including spheres, disks, stars, rods, wires, prisms, cubes and right bipyramids.6–10 More recently, silver nanoplates, as a two-dimensional anisotropic structure deviated from thermodynamics with sharp corners and edges, have particularly attracted our attention due to their fascinating optical properties11 and intensive use such as SERS detection,12 near-field optical probes,13 chemical and biological sensing.14 Recently, a number of various routes have been developed to tune the size and thickness for plate-like structure, such as templating methods,15 electrochemical synthesis,16 photo-induced process,17–19 and direct chemical reduction.20
The essential to form the extremely anisotropic plate-like structure is to overcome the thermodynamical tendency for minimizing the surface energy. A generic approach is to guarantee the slow formation rate for the nuclei and growth thus it could develop into the seed with stacking faults and covered by {111} facets at the top and bottom surfaces.8 To achieve this goal, controlling kinetics is generally necessary.21 One method is using a weak reducing agent to slow down the reduction rate. For example, Ag triangular nanoplates were produced by controlling the ratio of polyvinylpyrrolidone (PVP) whose hydroxyl end groups were found to serve as a grand weak reducing agent, it could also ensure the crystal growth slow enough.22 Another method is to introduce an oxidation procedure into the reduction. It had been demonstrated that the suitable utilization of the oxidative power of H2O2 could produce silver nanoplates through the formation of planar twinned defects and elimination of other less stable structure.23 In addition to employing of different reducing agents and oxidant, another strategy is to realize preferential anisotropic growth through selectively capping agents like surfactant, polymer, or small molecules, they could bind to a specific nanocrystallic facet, and simultaneously change the migration rate of that facet during nanocrystallic growth, resulting in plate-like structure.
However, among all morphology-tuned approaches, employ of foreign metal ions had been well-documented in literature of silver nanocrystal synthesis. For example, the impact of Cu+ ions or Cu2+ ions chloride in the silver polyol synthesis had been reported. It was found that ethylene glycol (EG) could reduce the Cu2+ to Cu+, which effectively prevented oxygen atoms from absorbing on the surface of silver nanoparticles, while Cl− controlled the saturation of free Ag+ and slowing the growth of seeds down, finally silver nanowires structure was generated.24 In the present work, we systematically investigated the influence of various foreign metal ions on the nucleation and growth of silver nanocrystals in benzyl alcohol (BnOH) based synthetic system. We found that, distinctively, the presence of Al3+ ions or Fe3+ ions can successfully lead to the formation of silver nanoplates.
In a typical solvothermal synthesis, silver trifluoroacetate (CF3COOAg) and PVP (Mw ≈ 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) were dissolved in the reaction solution, in which BnOH was used as the solvent and mild reducing agent. Different metal ions such as Mg2+, Al3+, Fe3+, Ni2+, Zn2+, Zr4+, etc. were added into the solution, and the total volume was fixed at 5 ml. The mixture was stirring for 10 min in the air at room temperature. Afterward, the solution was heated to 120 °C for 6 h (detailed information about the procedures can be found in the ESI†).
000) were dissolved in the reaction solution, in which BnOH was used as the solvent and mild reducing agent. Different metal ions such as Mg2+, Al3+, Fe3+, Ni2+, Zn2+, Zr4+, etc. were added into the solution, and the total volume was fixed at 5 ml. The mixture was stirring for 10 min in the air at room temperature. Afterward, the solution was heated to 120 °C for 6 h (detailed information about the procedures can be found in the ESI†).
The TEM images of final obtained silver nanocrystals are shown in Fig. 1. As displayed in Fig. 1a, without any foreign metal ions, the final product was quasi-spherical nanoparticles, whose size is 20–40 nm. Fig. 1b–d display the nanostructures synthesized with the described metal nitrates. It was evident the addition of Fe(NO3)3·9H2O or Al(NO3)3·9H2O could facilitate the formation of Ag nanoplates, with the yield to be 40% and 20%, respectively. Contrasting the shape and size, it is obvious to notice Al(NO3)3·9H2O leading to more uniform triangular plate nanostructure while the Fe(NO3)3·9H2O leading to diverse structures including nanodisks and triangular nanoplates.
Fig. 1d illustrates that TEM images in the presence of Mg(NO3)3·6H2O, with Ag nanoparticles obtained.
The X-ray diffraction (XRD) pattern of nanoparticles generated in different condition (as marked) was also carried out to identify the composition and purity, the results are shown in Fig. 2. Using the face-centered cubic (fcc) structured reference pattern JCPDF: 87-0720 (ref. 24), we indexed the peaks from left to right, they were {111}, {200}, {220} and {311}. The identification of the element composition was prepared with electron probe X-ray micro-analysis (EPMA) and plasma atomic emission spectroscopy (ICP-AES), as shown in Table 1. The molar ratios between Ag, Al and Fe were almost unanimously, and we suggest that little amount of Al in EPMA was induced by the Lα1 diffraction peak at 90.265 mm, which is similar to the Kα1 diffraction peak at 90.629 mm. This analysis shows that the final sample contained no Al or Fe element.
 | ||
| Fig. 2 XRD patterns of the Ag NCs obtained in the presence of PVP and different salts, as marked, Al corresponds to Al(NO3)3·9H2O and Fe corresponds to Fe(NO3)3·9H2O. JCPDF: 87-0720. | ||
| Additive | Analyzed molar ratios (Ag–Al–Fe) by EPMA | Analyzed molar ratios (Ag–Al–Fe) by ICP-AES |
|---|---|---|
| PVP | 0.995![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.005 0.005![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
| PVP + Al | 0.994![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.006 0.006![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
| PVP + Al + Fe | 0.993![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.007 0.007![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
| PVP + Fe | 0.994![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.006 0.006![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
To exclude the impact of anions contained in metal salts, we also repeated similar experiments with sulfates, acetates, etc. The TEM graphics of products in the presence of Fe2(SO4)3 and Al2(SO4)3·18H2O are shown in Fig. S1,† the Ag nanoplates can be both notably observed. From the above experiments, it can be inferred that the Fe3+ ions and Al3+ ions have an specific effect on the nucleation and growth process of silver nanocrystals, resulting in the formation of silver plate-like structure.
A systematic and detailed study was carried out to illuminate the effect of Fe3+ and Al3+ on the Ag NCs nucleation and growth. Firstly, the concentration of metal ions was found to significantly affect both the morphology and the yield of Ag nanoplates. For the Al3+ ions, when the molar mass of Al3+ ions was increased from 0.00625 mmol (Fig. 3a) to 0.025 mmol (Fig. 3b), the yield of triangular nanoplates raised from ca. 3% to ca. 15%. As the concentration up to 0.05 mmol, the yield of nanoplates can reach to 70% (Fig. 3c). Considering the addition of Fe3+ ions, when the concentration of Fe3+ ions was increased in the similar way (0.01875 mmol in Fig. 3e, 0.025 mmol in Fig. 3f and 0.05 mmol in Fig. 3g), the productivity of nanoplates dropped gradually, from ca. 45% to ca. 12%. We attempted to decrease the concentration of Fe3+ ions in the solution. It was found that when the concentration was as low as 0.0025 mmol, the yield with Fe3+ ions could reach to 70%, as shown in Fig. S2a.† The average edge length of the triangular plates is about 200 nm (Fig. S2b†) with various curvatures (Fig. S2a†).
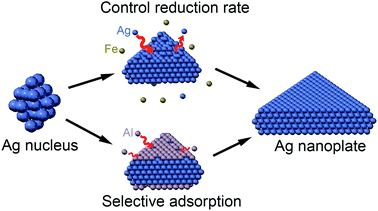
From the experimental results obtained, we discovered that Fe3+ ions and Al3+ ions have a significant impact on the morphology of Ag NCs and could facilitate the plate-like nanostructure with PVP as stabilizer. By controlling their concentration and ratios in the reaction solution, we can successfully synthesize high-yielding silver triangular nanoplates. Comparing the concentration–productivity curve of Al3+ ions system and Fe3+ ions system displayed in Fig. 3d and h, we found that their concentration alteration have totally different effect, two different mechanism have been proposed then. As shown in Fig. 3a–c, it is obvious that the productivity of Ag nanoplates could be improved through improving the concentration of Al3+ ions in reaction solution. We speculated that Al3+ ions could play as a capping agent, which preferentially adhere to {111} facets. With increasing concentration, more {111} facets have been block, then the growth rate of other facets like {100} was enhanced, finally Ag nanoplates would be synthesized. Further researches would be carried out on the mechanism. In the previous researches, Fe3+ ions were usually used to promote the formation of Ag nanowires in the polyol reduction of silver nitrate.25 Based on our observation, the addition of Fe3+ has promoted the formation of plate-like structure. The productivity of nanoplates is increased with the decrease of the concentration of Fe3+ ions, and the amount of the final structures of nanodisks or truncated nanoplates are considerable. It can be hypothesized that Fe3+ could oxidize Ag0 back to Ag+, thus reducing the super saturation of Ag atoms, therefore the kinetics during the nucleation and growth process had been altered. However, as the concentration of Fe3+ increases, the ratio of nanoplates in final product dropped. It can be inferred that high concentration of Fe3+ could promote the benzyl alcohol to reduce Fe3+ to Fe2+, which could in turn remove oxygen from the surface of Ag atoms,25 then more multiply twinned nanoparticles were formed. The illustration of the mechanism underlying the influence of these two ions is displayed in Fig. 4.
To further improve the yielding of plate-structured Ag NCs, we tried to add Al3+ ions and Fe3+ ions simultaneously, which combined the controlling kinetic and special absorption together. Fig. 5a shows the typical transmission electron microscopy (TEM) images of Ag nanocrystallines in the presence of Fe(NO3)3·9H2O and Al(NO3)3·9H2O. From which, we can obviously see that the final products are mainly triangular plates, whose size was ranging from 100 nm to 200 nm and the productivity of the nanoplates has reached up to 80%. Detailed characterization of the nanostructure obtained by the combination of Al3+ and Fe3+ ions was exhibited employing the high-resolution TEM and selected area electron diffraction (SAED) approaches. As shown in the inset of Fig. 5b, a typical electron diffraction (ED) pattern was recorded by directing the electron beam perpendicular to the top faces of an individual nanoplate (Fig. 5b). The six-fold rotational symmetry of the diffraction spots implies that the triangular faces are actually presented by {111} planes. Three sets of spots can be indentified based on the d-spacing: the spots (marked with the circle) with the strongest intensity could be indexed to the {220} reflections of fcc silver with a responding lattice spacing of 1.441 Å. The inner spots (marked with the triangle) with a weaker intensity is believed to be resulted from the formally forbidden 1/3{422} reflections and the outer set is related to the {311} planes of fcc silver. The inset in Fig. 5c is the high-resolution TEM image of the nanoplate recorded from the side face of an individual nanoplate. The red fringes could be indexed to {111} reflections, while the lattice spacing is measured to be 2.31 Å. The green fringes could be indexed to {200} reflection. These above assignment is consistent with some earlier researches, in which nanoplates are reported to be bounded by two {111} planes as the top and bottom faces and a mix of {100} and {110} planes as the side faces.7
Conclusions
In summary, we systematically investigate the effect of foreign metal ions on the formation of Ag nanocrystals. In our developed BnOH-based synthetic system, Al3+ ions or Fe3+ ions could lead to the produce of silver nanoplates, while Ag nanoparticles were obtained in the presence of other metal ions (Mg2+, Al3+, Fe3+, Ni2+, Zn2+, Zr4+, etc.). We proposed that Al3+ ions promote the nanoplates through preferential adsorption while Fe3+ ions alter kinetics during reduction. By the assistance of the improved understanding of the roles of foreign metal ions, the investigation in present work allows us to shed new light on the underlying mechanism of the formation of structural architectures of silver nanoparticles.Acknowledgements
The work was financially supported by the National Natural Science Foundation of China (51305225, 51321092), the National Key Basic Research Program of China (2013CB934204).Notes and references
- V. Voliani, F. Ricci, S. Luin and F. Beltram, J. Mater. Chem., 2012, 22, 14487–14493 RSC.
- V. Voliani, G. Signore, O. Vittorio, P. Faraci, S. Luin, J. Peréz-Prieto and F. Beltram, J. Mater. Chem. B, 2013, 1, 4225–4230 RSC.
- M. Liu, M. Leng, C. Yu, X. Wang and C. Wang, Nano Res., 2010, 3, 843–851 CrossRef CAS.
- Y. Sun and R. Qiao, Nano Res., 2008, 1, 292–302 CrossRef CAS.
- F. Xie, J. S. Pang, A. Centeno, M. P. Ryan, D. J. Riley and N. M. Alford, Nano Res., 2013, 6, 496–510 CrossRef CAS PubMed.
- D. Zhang, X. Liu and X. Wang, J. Mol. Struct., 2011, 985, 82–85 CrossRef CAS PubMed.
- K. E. Korte, S. E. Skrabalak and Y. Xia, J. Mater. Chem., 2008, 18, 437–441 RSC.
- Y. Xia, Y. Xiong, B. Lim and S. E. Skrabalak, Angew. Chem., Int. Ed., 2009, 48, 60–103 CrossRef CAS PubMed.
- L. Zhao, K. L. Kelly and G. C. Schatz, J. Phys. Chem. B, 2003, 107, 7343–7350 CrossRef CAS.
- A. Panáček, L. Kvitek, R. Prucek, M. Kolar, R. Vecerova, N. Pizurova, V. K. Sharma, T. Nevečná and R. Zboril, J. Phys. Chem. B, 2006, 110, 16248–16253 CrossRef PubMed.
- I. Pastoriza-Santos and L. M. Liz-Marzan, J. Mater. Chem., 2008, 18, 1724–1737 RSC.
- J. T. Zhang and X. M. Sun, J. Phys. Chem. B, 2005, 109, 12544–12548 CrossRef CAS PubMed.
- J. E. Millstone, G. S. Metraux and C. A. Mirkin, Small, 2009, 5, 646–664 CrossRef CAS PubMed.
- P. Alivisatos, Nat. Biotechnol., 2004, 22, 47–52 CrossRef CAS PubMed.
- E. C. Hao and J. T. Hupp, J. Am. Chem. Soc., 2002, 124, 15182–15183 CrossRef CAS PubMed.
- Y. G. Sun, Small, 2007, 3, 1964–1975 CrossRef CAS PubMed.
- R. C. Jin, C. A. Mirkin and G. C. Schatz, Science, 2001, 294, 1901–1903 CrossRef CAS PubMed.
- R. C. Jin, E. C. Hao and G. C. Schatz, Nature, 2003, 425, 487–490 CrossRef CAS PubMed.
- C. Xue, Angew. Chem., Int. Ed., 2007, 46, 2036–2038 CrossRef CAS PubMed.
- Y. J. Xiong, J. G. Wang and M. J. Kim, J. Mater. Chem., 2007, 17, 2600–2602 RSC.
- Y. J. Xiong, J. Y. Chen and Z. Y. Li, J. Am. Chem. Soc., 2005, 127, 17118–17127 CrossRef CAS PubMed.
- J. Y. Chen and Y. N. Xia, Angew. Chem., Int. Ed., 2005, 44, 2589–2592 CrossRef CAS PubMed.
- I. Washio and Y. D. Yin, Adv. Mater., 2006, 18, 1745 CrossRef CAS.
- Powder Diffraction File (PDF), International Center for Diffraction Data (ICDD), formerly JCPDS, Newtown Square PA, USA.
- B. Wiley and Y. N. Xia, Langmuir, 2005, 21, 8077–8080 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra01976h |
| This journal is © The Royal Society of Chemistry 2014 |