2,5-Dimethoxy 2,5-dihydrofuran crosslinked chitosan fibers enhance bone regeneration in rabbit femur defects†
Abstract
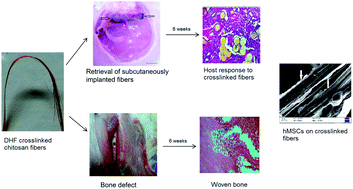
Chitosan fibers were fabricated via pH induced neutralization and precipitation in a 5 w/v% NaOH bath. Intermolecular covalent crosslinking of these fibers were performed through imine linkages between the glucosamine units of polymers and the dialdehyde groups of 2,5-dimethoxy-2,5-dihydrofuran at 60 °C, pH 2.2. The covalently crosslinked fibers demonstrated improved tensile strength, stiffness, hydrophobicity and higher stability against enzymatic degradation compared to uncrosslinked ones under wet conditions. The differences in the physico-chemical characteristics were reflected in protein adsorption which in turn facilitated higher cellular proliferation and adhesion to the crosslinked fibers. Osteogenesis of human bone marrow derived mesenchymal stem cells (hMSCs) was significantly higher on crosslinked fibers compared to the uncrosslinked ones as evidenced by higher alkaline phosphatase expression, calcium deposition and osteocalcin secretion. In vivo study performed subcutaneously in a rabbit model with crosslinked fibers revealed its ability to integrate with host tissues and showed differential extent of cellular infiltration and extracellular matrix production after specified periods of implantation. Further bone regeneration ability at the defect site filled with crosslinked fibers was evident by histological analysis. Thus, the study suggests that the imine crosslinked fibers could be used for bone tissue engineering applications.

 Please wait while we load your content...
Please wait while we load your content...