Open Access Article
Open Access ArticlePE/PEO blends compatibilized by PE brush immobilized on MWNTs: improved interfacial and structural properties
Prasanna Kumar S. Murala,
Manish Singh Ranab,
Giridhar Madrasab and
Suryasarathi Bose*c
aCenter for Nano Science and Engineering, Indian Institute of Science, Bangalore-560012, India
bDepartment of Chemical Engineering, Indian Institute of Science, Bangalore-560012, India
cDepartment of Materials Engineering, Indian Institute of Science, Bangalore-560012, India. E-mail: sbose@materials.iisc.ernet.in; Tel: +91-80-22933407
First published on 25th March 2014
Abstract
Polyolefin based blends have tremendous commercial importance in view of their exceptional properties. In this study the interface of a biphasic polymer blend of PE (polyethylene) and PEO (polyethylene oxide) has been tailored to reduce the interfacial tension between the phases and to render finer morphology. This was accomplished by employing various strategies like addition of maleated PE (PE grafted maleic anhydride), immobilizing PE chains, ex situ, onto MWNTs by covalent grafting, and in situ grafting of PE chains onto MWNTs during melt processing. Multiwalled nanotubes (MWNTs) with different surface functional groups have been synthesized either a priori or were facilitated during melt mixing at higher temperature. NH2 terminated MWNTs were synthesized by grafting ethylene diamine (EDA) onto carboxyl functionalized carbon nanotubes (COOH–MWNTs) and further, was used to reactively couple with maleated PE to immobilize PE chains on the surface of MWNTs. The covalent coupling of maleated PE with NH2 terminated MWNTs was also realized in situ in the melt extruder at high temperature. Both NH2 terminated MWNTs and the in situ formed PE brush on MWNTs during melt mixing, revealed a significant improvement in the mechanical properties of the blend besides remarkably improving the dispersion of the minor phase (PEO) in the blends. Structural properties of the composites were evaluated and the tensile fractured morphology was assessed using scanning electron microscopy.
Introduction
Blending of polymers is a versatile tool to achieve targeted properties with the existing polymers.1,2 In general, most polymeric blends are often thermodynamically immiscible in nature due to a small gain in entropy on mixing. The final morphology of the blends is contingent on the composition, viscosity ratio, interfacial tension, processing etc., resulting in a wide variety of microstructures such as matrix-droplet, fibrillar, lamellar or continuous. A compatibilizer like a block or a graft co-polymer is often added to reduce the interfacial tension between the constituents and to generate a finer morphology.3–5 For instance, Hashmi et al.,6 showed that the addition of styrene–butadiene–styrene (SBS) copolymer to polypropylene/polystyrene blends exhibited a finer morphology with respect to blends without SBS. Meltzer et al.,7 observed that addition of maleated PE to PE/PEO blend led to a significant refinement in morphology due to reactive compatibilization between maleic anhydride (MAH) and the hydroxyl ends of PEO.Polyolefins represent a class of important commodity polymers because of its good processability, low cost and good chemical resistance and hence, has tremendous commercial importance. Current research focuses to develop polyolefin based new materials with desired performance in view of its commercial importance. However, due to inherent immiscibility with other macromolecules, the desired properties are often not realized by mere blending. Further, the nonpolar nature of the polyolefins add to the challenge of compatibilizing polyolefinic blends. Therefore, various methods have been explored in the recent past to compatibilize polyolefinic blends.
The different morphologies generated during melt processing of polymer blends can be tailored to explore various strategic applications. Among the different morphologies, the matrix-droplet type offer a unique route to design substrates for separation technology. Polymeric membranes, in general, are derived from phase inversion process, thermally induced phase separation (TIPS), track etching or by solvent free methods like stretching melt-cast polymer films etc. While these methods have their own merits and demerits, an alternative route in designing polymer based membranes, which has recently attracted lot of attention, is by selectively etching one of the phases from a bi-phasic blend.7,8
Recent studies report the key role of nanoparticles (NPs) in compatibilizing binary blends. However, the underlying mechanism is significantly different from the classical approach (i.e. block/graft copolymer) of compatibilizing binary blends. Many factors such as surface free energy of the component, melt viscosity, processing parameters etc. govern the localization of NPs in a binary blend. Besides rendering finer microstructures, the NPs also offer unique functional properties which make them a potential candidate in designing polymer based nanocomposites. Among the NPs, carbon nanotubes (CNTs) have attracted a great deal of interest owing to their exceptional properties. While the majority of the work reported till date focuses on the electrical, mechanical and thermal properties of the composites, their key role in stabilizing binary blends has also been realized recently.
Therefore, in this study, we have determined the mechanical properties and dispersion of the phases in the presence of functionalized multiwalled nanotubes (MWNTs). The mechanical properties and the morphology of the blends were determined. We have attempted to reduce the interfacial tension between the phases by employing different strategies. These include the addition of PE grafted maleic anhydride, ex situ immobilizing PE chains on to MWNTs and in situ grafting of PE chains on to amine functionalized MWNTs. We have compared the various strategies and determined the blend that exhibits enhanced mechanical properties and shows increased dispersion of PEO in PE. The reasons for these observations are also discussed in detail.
Experimental
Materials
Low density polyethylene (PE) (melt flow index of 25 g per 10 min and density of 0.925 g cm−3), polyethylene oxide (PEO, Mv = 400![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000), maleated polyethylene (PE grafted with maleic anhydride), and ethylene diamine (EDA) were obtained from Sigma Aldrich. The pristine MWNTs (p-MWNTs, NC 7000 of 90% purity and 1.5 μm long) and acid functionalized MWNTs (COOH–MWNTs, NC 3150 of 95% purity and <1 μm long) were obtained from Nanocyl, Belgium. Solvents like tetrahydrafuran (THF), xylene, and thionyl chloride (SOCl2) were obtained from Merck, India and were used without further purification.
000), maleated polyethylene (PE grafted with maleic anhydride), and ethylene diamine (EDA) were obtained from Sigma Aldrich. The pristine MWNTs (p-MWNTs, NC 7000 of 90% purity and 1.5 μm long) and acid functionalized MWNTs (COOH–MWNTs, NC 3150 of 95% purity and <1 μm long) were obtained from Nanocyl, Belgium. Solvents like tetrahydrafuran (THF), xylene, and thionyl chloride (SOCl2) were obtained from Merck, India and were used without further purification.
Synthesis of NH2 terminated MWNTs
NH2 terminated MWNTs were synthesized from carboxyl (–COOH) functionalized MWNTs using EDA; a convenient method that has been widely applied in functionalizing MWNTs. Typically, 800 mg of MWNT–COOH was suspended in THF and was mixed with 80 mL of SOCl2 in a 250 mL round bottomed flask. The mixture was then stirred at 80 °C for 24 h in presence of EDA. The powder was isolated by vacuum filtration and was washed with anhydrous THF to remove excess SOCl2. This purification cycle was repeated couple of times to ensure complete removal of excess SOCl2 and the final product was vacuum dried for 24 h at 80 °C.Immobilizing PE on the surface of MWNTs
PE grafted MWNT were prepared by grafting maleated PE on to NH2 terminated MWNTs via an amidation reaction between the amine groups of NH2 terminated MWNTs and the maleic anhydride groups of maleated PE. In a typical reaction, 50 mg of NH2 terminated MWNTs was initially suspended in 10 mL of xylene. The mixture was sonicated for half an hour to exfoliate the nanotubes. 120 mg of maleated PE was then added to this mixture and was refluxed at 100 °C under N2 atmosphere. The mixture was then filtered with a 0.22 μm PTFE membrane. The collected powder was extracted with 200 mL of boiling xylene for 48 h to remove un-grafted maleated PE, and dried at 90 °C under vacuum overnight, thus immobilizing PE chains on the surface of MWNTs (PE-g-MWNTs).Characterization of NH2 terminated MWNTs and PE-g-MWNT
X-ray Photon Scattering (XPS) scans were recorded on a Kratos Analytical instrument using Al monochromatic source (1.486 keV) and the Fourier transform infrared (FTIR) spectra were recorded on a Thermo Nicolet 6700 in the wavelength range of 4000–400 cm−1 using KBr pellets. TGA was carried out under N2 atmosphere with a Pyris Diamond analyzer at a heating rate of 10 °C min−1. Transmission Electron Microscopy (TEM) images were acquired using Tecnai G2 F30 at 300 kV.Preparation of compatibilized blends
Blends with and without compatibilizers (maleated PE), and MWNTs (p-MWNT, NH2 terminated MWNTs and PE-g-MWNT) were prepared by melt mixing under N2 atmosphere in a laboratory scale twin screw extruder (Polylab, Thermohaake Minilab II) at 150 °C and 60 rpm for 20 min. The mini extruder has a recirculation channel which ensures complete mixing for desired time before the material can be taken out. Prior to melt mixing all the samples were vacuum dried overnight.Characterization of blends
For tensile properties, dumbbell shaped specimens were prepared by compression molding at 150 °C. The tensile tests were done on Instron Universal Testing Machine at room temperature with crosshead speed of 5 mm min−1. Morphological analysis for tensile fractured samples were assessed using field emission scanning electron microscopy (FE-SEM) on a ULTRA 55, FESEM, Carl Zesis with accelerating voltage of 5 kV. The morphology of the as pressed films were analyzed using SEM. Prior to SEM, the films were etched with cold sterilized distilled (DI) water to remove the PEO phase.The melting temperature (Tm), crystallization temperature (Tc) and % crystallinity (Xc) of PE (normalized with respect to weight and weight fraction) was measured using a Mettler Toledo DSC instrument with a heating and cooling rate of 10 °C min−1. The degree of crystallinity of PE phase was calculated from the heat of fusion of second heating cycle. The heat of fusion (ΔHm) of PE phase was normalized to the fraction of polymer present in the blends. The degree of crystallinity (Xc) of PE phase was determined from the ratio of normalized heat of fusion (ΔHm, norm) to the heat of fusion of 100% crystalline PE, (ΔH0f), which was taken as 293 J g−1.9
Results and discussion
Synthesis and characterization of NH2 terminated MWNTs and PE-g-MWNT
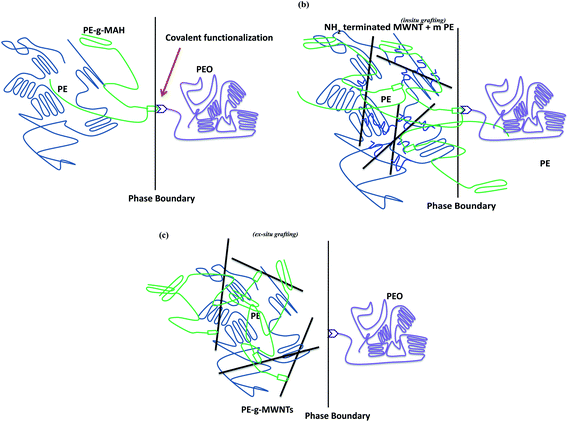
As shown in Scheme 1, a two-step procedure was adopted to prepare NH2 terminated MWNTs. It consisted of NH2 attachment on to MWNT surface by acylation with thionyl chloride (SOCl) at 65 °C for 24 h. This was followed by amidation of the resulted MWNT–COCl with an excess of EDA at 80 °C for 48 h to complete the functionalization process. The excess of EDA was then washed with anhydrous THF and filtered using 0.22 μm PTFE membrane. In the second step, maleated PE was coupled to NH2 terminated MWNTs through the formation of amide groups, yielding PE-g-MWNTs, as shown in Scheme 1.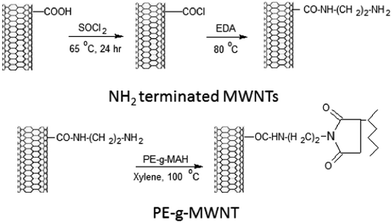 | ||
| Scheme 1 Immobilizing PE on to MWNTs via reactive coupling of maleated PE with NH2 terminated MWNTs. | ||
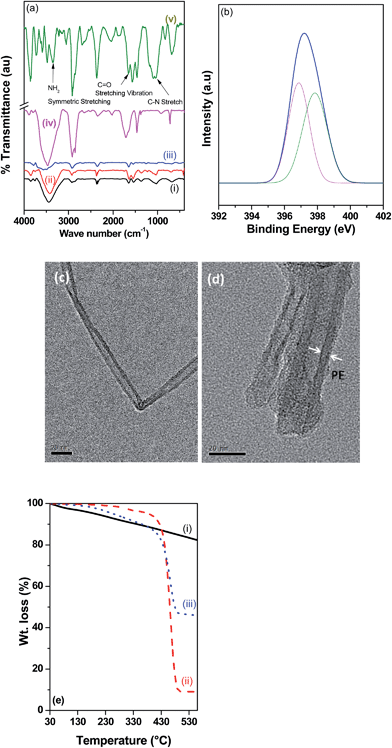
The direct evidence for EDA functionalized MWNTs and successful grafting of PE was provided by FTIR and XPS. Fig. 1a and b illustrates the FTIR and XPS scans of NH2 terminated MWNTs and PE-g-MWNTs. A broad peak at 3424 cm−1 in the FTIR spectra indicates the presence of (–OH) hydroxyl groups in MWNT–COOH. Upon coupling with EDA, a peak appears at 1653 cm−1 indicating the amide carbonyl (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) stretch. Similar observations were reported earlier.10,11 The presence of C–N stretching vibrations at 1044 cm−1 and the symmetric stretching at 3359 cm−1 confirms the presence of saturated primary amine on the MWNT surface. In the FTIR spectrum of PE-g-MWNTs, peaks at 1375 cm−1 and 1455 cm−1 are assigned to bending vibration of CH3, while peak at 1719 cm−1 indicates C
O) stretch. Similar observations were reported earlier.10,11 The presence of C–N stretching vibrations at 1044 cm−1 and the symmetric stretching at 3359 cm−1 confirms the presence of saturated primary amine on the MWNT surface. In the FTIR spectrum of PE-g-MWNTs, peaks at 1375 cm−1 and 1455 cm−1 are assigned to bending vibration of CH3, while peak at 1719 cm−1 indicates C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration of maleic anhydride. In addition, peak at 2916 cm−1 shows stretching vibrations of PE. A shift of C
O stretching vibration of maleic anhydride. In addition, peak at 2916 cm−1 shows stretching vibrations of PE. A shift of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration from 1709 to 1656 cm−1 confirms the covalent bonding of maleated PE on to MWNT surface.12,13
O stretching vibration from 1709 to 1656 cm−1 confirms the covalent bonding of maleated PE on to MWNT surface.12,13
Functionalization of MWNTs with EDA is further confirmed by XPS. The presence of N1s peak in functionalized MWNTs indicates the presence of amine. Furthermore, N1s peak of NH2 terminated MWNTs was fitted to deconvolute the two species (see Fig. 1b). The lower binding energy (BE) peak at 396.9 eV is attributed to terminal amine and the higher BE peak at 397.8 eV could be attributed to the amide nitrogen. The latter showed higher BE due to its electropositive nature and manifests the electron withdrawing nature of the carbonyl group.14,15
In general, immobilization of macromolecular chains on MWNTs can influence their thickness, which can be characterized by TEM and the fraction of polymer tethered on the MWNT surface can be evaluated by TGA. Fig. 1c–d shows the TEM micrographs of MWNT–COOH and PE-g-MWNTs. The latter showed a layer of polymer on the MWNTs as manifested from its increased diameter. This can result in improved dispersion of MWNTs in the PE phase of the blends. Fig. 1e shows the TGA scans for MWNT–COOH, maleated PE and PE-g-MWNT. At 550 °C, the relative weight losses for MWNT–COOH, maleated PE and PE-g-MWNTs are 17%, 91 and 54 wt% respectively. Large weight loss of PE-g-MWNTs is attributed to the thermal degradation of attached PE polymer chains. Thus confirming the maleated PE being grafted successfully on MWNTs.13
Structure–property relationship in PE-g-MWNTs compatibilized PE/PEO blends
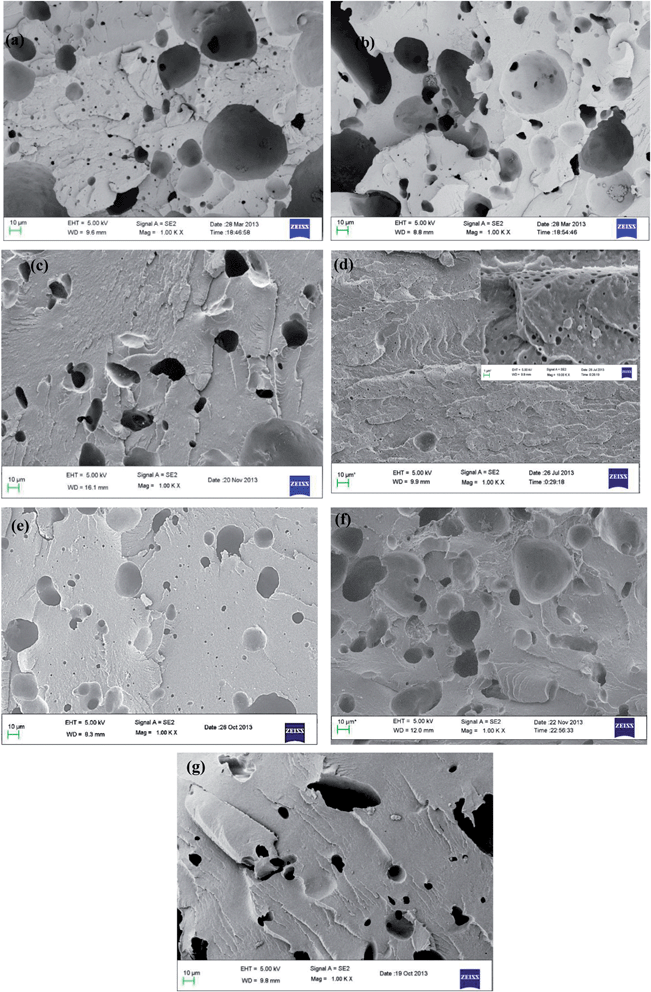
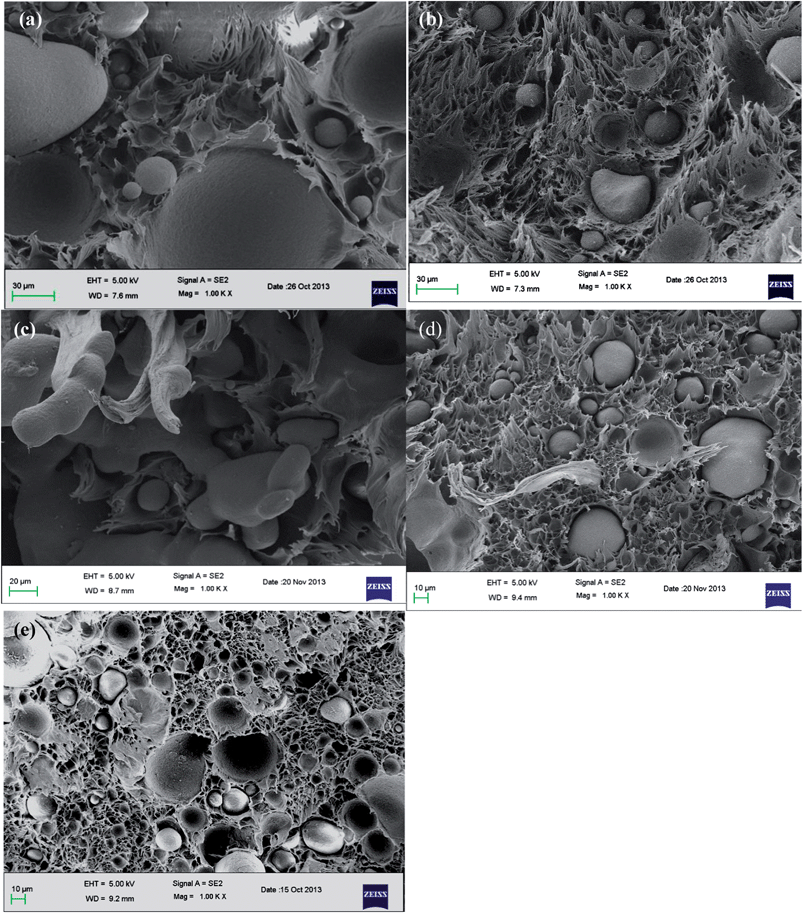
An efficient route to tailor the interface in binary polymer blends is by adding a compatibilizer. Next to the classical block copolymers, recent studies have revealed the key role of NPs in compatibilizing binary blends as discussed in the introduction section. In our earlier work, we have shown the potential of MWNTs in suppressing the coalescence of PMMA droplets in the PαMSAN matrix and the refinement of bi-continuous structures in PA6/ABS blends. Although, MWNTs render finer morphology irrespective of its localization in the blends, the mechanism is very different from the classical block copolymer route. It is understood from one of our previous work16 that MWNTs localize in the thermodynamically less favored phase (PE) in PE/PEO blends which is driven by the flow characteristic of the components (the melt viscosity of PEO is significantly higher than PE). The key role of MWNTs as a compatibilizer may not be realized in PE/PEO blends due to lack of interfacial adhesion of MWNTs with the non-polar PE phase. Hence, in this study, we adopted two different approach to compatibilize PE/PEO blends: by in situ and ex situ immobilization of PE chains on to MWNTs as discussed before. However, both in situ and ex situ grafting of PE chains on to MWNTs can serve as an effective compatibilizer in PE/PEO blends. Recall, that the in situ grafting of PE chains on to MWNTs was realized by reactive coupling of maleated PE with NH2 terminated MWNTs during melt-mixing and the ex situ was realized by grafting maleated PE with NH2 terminated MWNTs a priori before melt mixing. Blends with pristine MWNT (p-MWNTs) was also prepared and investigated for their compatibilizing effects in PE/PEO blends.The improved compatibility is revealed by SEM and is illustrated in Fig. 2a–g. To identify the minor phase, the specimens for SEM were immersed in DI water so that the dispersed PEO phase appears as holes in the PE matrix. Blends of PE/PEO are immiscible in nature and typically exhibit coarse morphology (see Fig. 2a). Clearly, the PEO holes in PE/PEO blends with different MWNTs (Fig. 2b–c) are much smaller than those of the neat blends, indicating that PEO is more finely in dispersed PE/PEO/MWNT blends. Interestingly, with addition of 3 wt% maleated PE in PE/PEO blends, the size of the dispersed PEO phase drastically reduced (see Fig. 2d and the inset of Fig. 2d) in striking contrast to the neat blends. This reduction in the PEO droplets clearly indicate the role of maleated PE in compatibilizing PE/PEO blends by possibly reducing the interfacial tension between PE and PEO.17 More interestingly, the in situ grafting of maleated PE on to NH2 terminated MWNTs, through amidation during processing, has led to finer dispersion of PEO droplets in PE (see Fig. 2f) and which is not realized with p-MWNTs (see Fig. 2e). A schematic description of the compatibilizing mechanism of maleated PE and in situ grafting of PE on to MWNTs is displayed in Scheme 2. Due to high interfacial tension, macrophase separation exists in uncompatibilized PE/PEO blends. With the introduction of maleated PE and PE-g-MWNTs, the interface is modified thereby reducing the unfavorable interactions. Consequently, the ex situ grafting of PE chains on to MWNTs led to better dispersion of MWNTs in the PE phase and the combination of maleated PE further resulted in a finer dispersion of PEO droplets in the blends (see Fig. 2g).
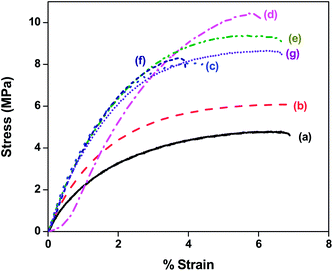
The reinforcing effect of MWNTs, maleated PE and PE-g-MWNTs is evaluated using tensile tests. Fig. 3 demonstrates the typical stress–strain curves for uncompatibilized and compatibilized by maleated PE and PE-g-MWNTs. Both PE and PEO show ductile behavior during uniaxial tensile test (not shown here). The control PEO exhibit a yield point followed by cold drawing and strain hardening (not shown here) whereas, the 70/30 PE/PEO blends exhibit a typical brittle failure with poor ultimate tensile strength and modulus. This is due to poor interfacial adhesion between PE/PEO. The corresponding tensile fracture morphology is shown in Fig. 4a. Clearly, the debonding at the interface is a cause of premature failure, thus lowering the mechanical properties with respect to the components (see Table 1). This is discussed in more details in the next section. A classical route to reduce the interfacial tension and improve the stress transfer at the interface is by compatibilizing.18 In the present work, we employed two different approaches; in situ and ex situ grafting of PE chains on to MWNTs. The use of maleated PE showed an increase of 109% in the ultimate tensile strength and 106% in the Young's Modulus (YM) in the blends. This dramatic improvement in the mechanical properties is attributed to the specific interactions between maleic anhydride (MAH) and the terminal hydroxyl (OH) groups in PEO.7
| Sample | Ultimate tensile strength (MPa) | Tensile modulus (MPa) | Elongation at break (%) |
|---|---|---|---|
| Neat 70/30 blend | 5 ± 0.6 | 173 ± 8.0 | 7.0 ± 0.5 |
| With 1 wt% p-MWNTs | 6 ± 0.1 | 268 ± 7.6 | 6.9 ± 1.1 |
| With 1 wt% NH2-t-MWNTs | 8 ± 0.1 | 481 ± 20 | 4.4 ± 0.3 |
| With 3 wt% PE-g-MAH | 10 ± 0.1 | 356 ± 4.6 | 4.8 ± 0.8 |
| With 3 wt% PE-g-MAH and 1 wt% p-MWNT | 9 ± 0.6 | 399 ± 8.7 | 6.0 ± 0.8 |
| With 3 wt% PE-g-MAH and 1 wt% NH2-t-MWNTs | 8 ± 0.2 | 496 ± 9.2 | 3.9 ± 0.5 |
| With 3 wt% PE-g-MAH and 1 wt% PE-g-MWNT | 8 ± 0.2 | 421 ± 6.5 | 6.5 ± 1.1 |
Mechanical properties of the blends largely depend on the phase morphology and interfacial adhesion between the constituents. It is envisaged that the elongation at break is an important parameter to judge the interfacial adhesion between the phases, whereas the tensile strength is an indication of finer morphology, domain size and size homogeneity.19 Incorporation of p-MWNTs and NH2 terminated MWNTs showed an increase in YM by 55% and 178% respectively; and in the ultimate tensile strength by 20% and 60% respectively. Although the MWNTs exhibit a reinforcement effect in the blends but the elongation at break is greatly sacrificed. This observation is a clear mandate to the fact that premature failure originates from the CNT aggregates19 and due to lack of interfacial adhesion with the matrix (here PE) polymer. Interestingly, NH2 terminated MWNTs showed higher mechanical strength than the p-MWNT and can be attributed to possible interfacial interactions with PEO as well thereby acting as an interfacial modifier. In order to improve the interfacial adhesion and to tailor the interface, in situ grafting of maleated PE on to NH2 terminated MWNTs was facilitated during processing. The thus formed PE-g-MWNTs showed exceptionally high YM (186%) and ultimate tensile strength (60%) and more interestingly, retained the elongational properties. It is observed that for 1 wt% of PE-g-MWNT an increase of 78% in ultimate tensile strength and 143% in YM is recorded.
In order to support the observations above and to understand the mechanism of fracture, the tensile fractured morphology is investigated in detail here and is shown in Fig. 4a–e. The tensile fractured surfaces of the neat 70/30 PE/PEO blends reveal that PEO droplets is embedded in the PE matrix and with a weak interface. PE is a semicrystalline and ductile material, the lack of interfacial adhesion between PE and PEO caused an early failure with respect to the constituents. Peterlin20 proposed that the component which has low strength and ductility (here PE), comprises of stacks of parallel lamellae with fewer links in-between which in turn deform plastically and spreads to maximum compliance before fracture. The typical failure in ductile semicrystalline polymer comprises of fibrillar morphology in the final stage of deformation. Hence, the fibrillar morphology observed in Fig. 4b can be related with the fact that the PE phase shares the most load and due to poor interfacial adhesion, the stress transfer at the interface is relatively poor. Two interesting observations are noted here. Firstly, with the addition of maleated PE, the fibrils appear to be thinner with respect to the fractured surfaces of the neat blends manifesting the compatibilization rendered in the PE/PEO blends. Secondly, the fine dispersion of PEO phase in the PE matrix.19,21
Incorporation of MWNTs in the blends led to reduction in the fibrillar morphology again suggesting poor interfacial adhesion between the phases. This observation is also reflected in the elongational properties of the blends. It is now understood that both fibrillar morphology and finer distribution of PEO phase are important to obtain improved mechanical properties in PE/PEO blends. In addition, MWNTs improve the YM of the blend significantly in striking contrast to the improvement in ultimate tensile strength. The latter property is strongly contingent on the interfacial adhesion between the phases; however, the reinforcement effected by MWNTs is well realized by the improvements in YM. A combination of MWNTs and maleated PE could result in simultaneous improvement in YM and ultimate tensile strength provided PE grafts are formed on the surface of MWNTs during melt mixing. In order to realize this, we compared the mechanical properties of the blends with ex situ PE-g-MWNTs with that of the in situ formed PE grafts on to MWNTs during processing. Interestingly, although ex situ formed PE-g-MWNTs are relatively well dispersed in the PE phase, the in situ formed PE-g-MWNTs resulted in significant improvement in YM and ultimate tensile strength. These observations are also supported by the tensile fractured morphology where finer dispersion of PEO phase is well evident next to improved interfacial adhesion, as seen in Fig. 5 where in situ formed grafts were observed to bridge the phases.
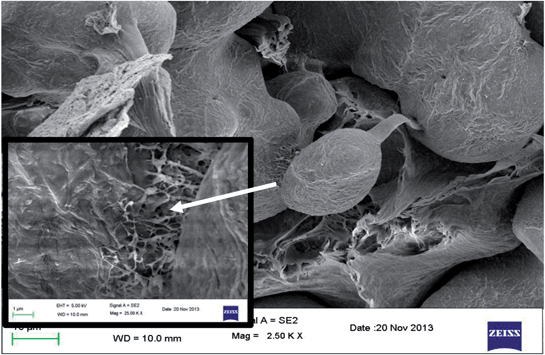 | ||
| Fig. 5 High resolution SEM image of tensile fractured surface of 3 wt% maleated PE and 1 wt% NH2 terminated MWNTs. Inset shows MWNT bridging the phases. | ||
Melting and crystallization behavior of the blends: effect of MWNTs
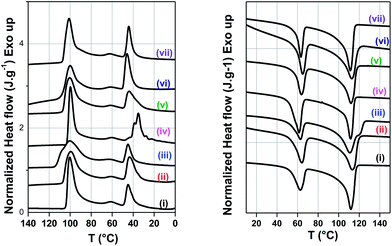
Fig. 6 shows the cooling (Fig. 6a) and the heating scans (Fig. 6b) of PE/PEO blends and the melting and crystallization temperatures for various blends investigated here are listed in Table 2. The corresponding exotherms of PE and PEO is well evident in the cooling scans of the blends; a typical characteristic of un-compatibilized blend.22 The crystallization temperatures (Tc) of PE and PEO are observed to be 100.4 °C and 44.8 °C, respectively. Two interesting observations draw our attention. Firstly, with addition of 3 wt% maleated PE in PE/PEO blends, the Tc of PEO has been delayed by ca. 10 °C with respect to neat blends while the Tc of PE remains changed. Secondly, we observed fractionated crystallization in PE/PEO blends in the presence of maleated PE. The melt interfacial reaction between maleated PE and the terminal hydroxyl group of PEO possibly has led to delayed crystallization in PEO.23 It is worth recalling that maleated PE has remarkably improved the dispersion of PEO in PE matrix (see Fig. 2d). It is envisaged that the heterogeneities are strongly contingent on the droplet size. For instance, the smaller droplets might contain less efficient heterogeneities and will only nucleate at the temperature when they become active.23 A moderate decrease in the melting temperature of PEO (see Fig. 6b) also suggests amorphous–amorphous miscibility in the presence of maleated PE. In the presence of MWNTs, no appreciable change is observed in the crystallization temperature of either PE or PEO. It is envisaged that CNTs act as hetero nucleating agent and influence the rate of crystallization in semicrystalline polymer.24,25 In this case, MWNTs or modified MWNTs did not show any appreciable change in the crystallization and melting temperature. Hence, it can be concluded that the observed changes in the structural properties are closely related with the strengthened interface on account of melt-interfacial interactions.| Sample | Melting temperature (Tm) (°C) | Crystallization temperature (Tc) (°C) | ||
|---|---|---|---|---|
| PE | PEO | PE | PEO | |
| Neat 70/30 blend | 112 | 63 | 100 | 45 |
| With 1 wt% p-MWNTs | 114 | 64 | 100 | 44 |
| With 1 wt% NH2-t-MWNTs | 111 | 63 | 101 | 45 |
| With 3 wt% PE-g-MAH | 111 | 61 | 100 | 35 |
| With 3 wt% PE-g-MAH and 1 wt% p-MWNT | 112 | 64 | 101 | 44 |
| With 3 wt% PE-g-MAH and 1 wt% NH2-t-MWNTs | 113 | 65 | 100 | 46 |
| With 3 wt% PE-g-MAH and 1 wt% PE-g-MWNT | 111 | 63 | 101 | 44 |
Conclusions
In order to render finer morphology in the blends, various routes for compatibilization such as addition of maleated PE (PE grafted maleic anhydride), immobilizing PE chains, ex situ, onto MWNTs by covalent grafting, and in situ grafting of PE chains on to MWNTs during melt processing were adopted. Blends with modified MWNTs revealed a significant improvement in the mechanical properties of the blend besides remarkably improving the dispersion of the minor phase (PEO) in the blends. Blends with in situ formed PE-g-MWNTs showed exceptionally high Young's modulus (186% higher with respect to neat blends) and ultimate tensile strength (60% as compared to neat blends) and more interestingly, retained the elongational properties.Acknowledgements
Department of Science and Technology (DSTO1150) and JATP is gratefully acknowledged for the financial support. We would like to thank MNCF, CeNSE for providing instrumentation facilities.References
- S. Bose, A. R. Bhattacharyya, A. R. Kulkarni and P. Pötschke, Electrical, rheological and morphological studies in co-continuous blends of polyamide 6 and acrylonitrile–butadiene–styrene with multiwall carbon nanotubes prepared by melt blending, Compos. Sci. Technol., 2009, 69, 365–372 CrossRef CAS PubMed.
- L. A. Utracki, Polymer Blends Handbook, Kluwer Academic Pub, 2002 Search PubMed.
- J. R. Dygas, B. Misztal-Faraj, Z. Florjańczyk, F. Krok, M. Marzantowicz and E. Zygadło-Monikowska, Solid State Ionics, 2003, 157, 249 CrossRef CAS.
- O. Valentino, M. Sarno, N. G. Rainone, M. R. Nobile, P. Ciambelli, H. C. Neitzert and G. P. Simon, Influence of the polymer structure and nanotube concentration on the conductivity and rheological properties of polyethylene/CNT composites, Phys. E, 2008, 40, 2440–2445 CrossRef CAS PubMed.
- M. Armand, J. M. Chabagno, M. Duclot, P. Vashista, J. N. Mundy and G. Shenoy, Fast Ion Transport in Solids, 1979 Search PubMed.
- S. A. Hashmi, A. Kumar, K. K. Maurya and S. Chandra, J. Phys. D: Appl. Phys., 1990, 23, 1307 CrossRef CAS.
- Y. L. Meltzer, Water-Soluble Polymers, Recent Developments, 1979 Search PubMed.
- S. D. Druger, A. Nitzan and M. A. Ratner, Phys. Rev. B: Condens. Matter Mater. Phys., 1985, 31, 3939 CrossRef CAS.
- S. Z. Cheng, Handbook of Thermal Analysis and Calorimetry: Applications to Polymers and Plastics, Elsevier, 2002 Search PubMed.
- B. K. Choi, Solid State Ionics, 2004, 168, 123 CrossRef CAS PubMed.
- K. C. Sobha and K. J. Rao, Solid State Ionics, 1995, 81, 145 CrossRef CAS.
- C. Chen, H. Pang, Z. Liu, Y.-B. Li, Y.-H. Chen, W.-Q. Zhang, X. Ji and J.-H. Tang, Enhanced foamability of isotactic polypropylene composites by polypropylene-graft-carbon nanotube, J. Appl. Polym. Sci., 2013, 130, 961–968 CrossRef CAS PubMed.
- C. Li, H. Deng, K. Wang, Q. Zhang, F. Chen and Q. Fu, Strengthening and toughening of thermoplastic polyolefin elastomer using polypropylene-grafted multiwalled carbon nanotubes, J. Appl. Polym. Sci., 2011, 121, 2104–2112 CrossRef CAS PubMed.
- B. X. Yang, K. P. Pramoda, G. Q. Xu and S. H. Goh, Mechanical Reinforcement of Polyethylene Using Polyethylene-Grafted Multiwalled Carbon Nanotubes, Adv. Funct. Mater., 2007, 17, 2062–2069 CrossRef CAS PubMed.
- B.-X. Yang, J.-H. Shi, K. P. Pramoda and S. H. Goh, Enhancement of the mechanical properties of polypropylene using polypropylene-grafted multiwalled carbon nanotubes, Compos. Sci. Technol., 2008, 68, 2490–2497 CrossRef CAS PubMed.
- P. K. Mural, G. Madras and S. Bose, Positive temperature coefficient and structural relaxations in selectively localized MWNTs in PE/PEO blends, RSC Adv., 2014, 4, 4943–4954 RSC.
- J. Borah and T. Chaki, Dynamic rheological, morphology and mechanical properties of compatibilized LLDPE/EMA blends, J. Polym. Res., 2011, 18, 907–916 CrossRef CAS.
- A. Leclair and B. D. Favis, The role of interfacial contact in immiscible binary polymer blends and its influence on mechanical properties, Polymer, 1996, 37, 4723–4728 CrossRef CAS.
- S. Bose, A. R. Bhattacharyya, L. Häußler and P. Pötschke, Influence of multiwall carbon nanotubes on the mechanical properties and unusual crystallization behavior in melt-mixed co-continuous blends of polyamide6 and acrylonitrile butadiene styrene, Polym. Eng. Sci., 2009, 49, 1533–1543 CAS.
- A. Peterlin, Molecular model of drawing polyethylene and polypropylene, J. Mater. Sci., 1971, 6, 490–508 CrossRef CAS.
- M. E. Villarreal, M. Tapia, S. M. Nuño-Donlucas, J. E. Puig and R. González-Núñez, Mechanical properties of polystyrene/polyamide 6 blends compatibilized with the ionomer poly(styrene-co-sodium acrylate), J. Appl. Polym. Sci., 2004, 92, 2545–2551 CrossRef CAS PubMed.
- H. Liu, T. Xie, Y. Zhang, Y. Ou and G. Yang, Crystallization behaviors of polypropylene/polyamide-6 blends modified by a maleated thermoplastic elastomer, Polym. J., 2006, 38, 21–30 CrossRef CAS.
- C. Yordanov and L. Minkova, Fractionated crystallization of compatibilized LDPE/PA6 blends, Eur. Polym. J., 2005, 41, 527–534 CrossRef CAS PubMed.
- S. Bose, A. R. Bhattacharyya, P. V. Kodgire and A. Misra, Fractionated crystallization in PA6/ABS blends: Influence of a reactive compatibilizer and multiwall carbon nanotubes, Polymer, 2007, 48, 356–362 CrossRef CAS PubMed.
- S. Bose, A. R. Bhattacharyya, L. Häußler and P. Pötschke, Influence of multiwall carbon nanotubes on the mechanical properties and unusual crystallization behavior in melt-mixed co-continuous blends of polyamide6 and acrylonitrile butadiene styrene, Polym. Eng. Sci., 2009, 49, 1533–1543 CAS.
| This journal is © The Royal Society of Chemistry 2014 |