Pyrophosphate selective recognition by a Zn2+ complex of a 2,2′-binaphthalene derivative bearing di(2-pyridylmethyl)aminomethyl groups in aqueous solution†
Shin-ichi
Kondo
*ab,
Yasunori
Nakadai
c and
Masafumi
Unno
*c
aDepartment of Material and Biological Chemistry, Faculty of Science, Yamagata University, Yamagata 990-8560, Japan. E-mail: kondo@sci.kj.yamagata-u.ac.jp; Fax: +81-23-628-4587; Tel: +81-23-628-4587
bInstitute for Regional Innovation, Yamagata University, Kanakame, Kaminoyama, Yamagata 999-3101, Japan
cDepartment of Chemistry and Chemical Biology, Graduate School of Science and Technology, Gunma University, Kiryu, Gunma 376-8515, Japan
First published on 12th June 2014
Abstract
We have synthesized a 2,2′-binaphthalene derivative 1 bearing di(2-pyridylmethyl)aminomethyl groups at 8- and 8′-positions. The receptor 1 formed a dinuclear Zn2+ complex in aqueous solution. The complex 1·2Zn2+ can selectively recognize the biologically important pyrophosphate anion and characteristic responses in both UV-vis and fluorescence spectroscopies were observed.
Introduction
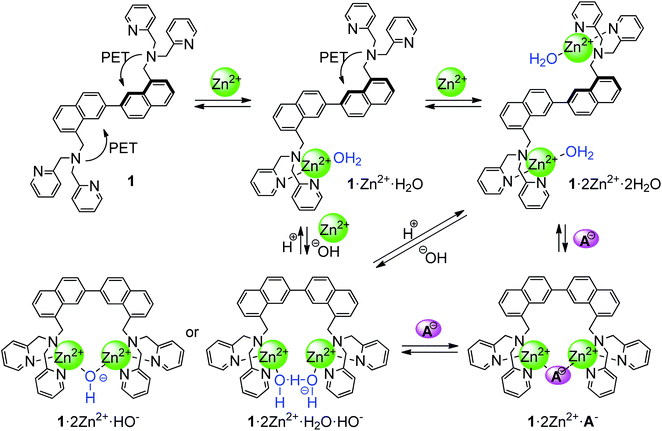
Artificial anion receptors have been continuously focused on in host–guest chemistry, however, the selective recognition of biologically important anions in an aqueous solution is still a challenging task.1 In particular, inorganic pyrophosphate (PPi) is a biologically important compound due to participation of bioenergetical and metabolic processes, and pyrophosphate level of patients should be monitored.2 In this regard, the detection of pyrophosphate is one of the urgent research areas in molecular recognition chemistry. Fluorescence chemosensors have widely been studied because of their high sensitivity and ease of operation.3 Anion recognition and sensing using Zn2+–di(2-pyridylmethyl)amine (Zn2+–DPA) functionalized receptors have been reported over the past decade.4 Indeed, several research groups have studied fluorescent detection of pyrophosphate by dinuclear complexes bearing a fluorophore as a signalling unit.5 Detection by UV-vis spectroscopy is another useful optical sensing technique, which requires more low-cost and simple instruments for measurements.6 However, there are only a few examples of pyrophosphate selective receptors by both fluorescence and UV-vis spectroscopies.5e,7 Ojida et al. reported turn on fluorescence sensing by a xanthene-based sensor bearing two DPA units of pyrophosphate and related compounds.5e In the absence of pyrophosphate, xanthene ring is reacted with the hydroxide ion to form a deconjugated structure, however, the conjugated xanthene structure is recovered by the addition of pyrophosphate. The structural change in the xanthene moiety shows UV-vis spectral changes with pyrophosphate. Yoon and co-workers reported that a zinc complex with Zinpyr-1, in which fluorescein was used as fluorophore, showed both UV-vis and fluorescence changes upon the addition of pyrophosphate, but the mechanism of the UV-vis changes is not clear.7We have designed 2,2′-binaphthalene-based receptors bearing cooperative recognition sites at 8- and 8′-positions as not only fluorophore but also chromophore by the restriction of rotation around the single bond between two naphthyl groups during a complexation with appropriate analytes.8 This conformational restriction induces a characteristic diminishment of UV-vis absorbance at around 310 nm by a predominant formation of the cisoid conformer of 2,2′-binaphthyl moiety in spite of lacking any conjugation between 2,2′-binaphthalene as a chromophore and the recognition sites. It should be mentioned that 2,2′-binaphthyl moiety is not reacted (unchanged) during recognition of analytes. Following these idea, we now report on the synthesis and recognition ability of 2,2′-binaphthalene 1 bearing di(2-pyridylmethyl)aminomethyl moieties at 8- and 8′-positions. The receptor formed the corresponding dinuclear Zn2+ complex and the complex showed selective UV-vis and fluorescence responses for biologically relevant pyrophosphate anion in aqueous solution.
Results and discussion
Synthesis of 8,8′-bis(bromomethyl)-2,2′-binaphthalene was carried out by the previously reported method.8b Substitution of the bromo groups with di(2-pyridylmethyl)amine in the presence of triethylamine in THF gave receptor 1 in good yield as shown in Scheme 1. The structure of 1 was fully confirmed by 1H, 13C NMR spectra as well as COSY, HMQC, and HMBC NMR techniques, HRMS, and elemental analysis.At first, the binding ability of 1 with Zn2+ was studied in 20% MeCN–buffer solution (v/v) due to low solubility of 1 in pure water. The UV-vis spectrum of 1 showed a broad peak around 312 nm ascribed to the π–π* transition of 2,2′-binaphthyl moiety. In neutral condition (20% MeCN–10 mM HEPES, pH 7.2), the absorbance at 312 nm was significantly decreased arising from the formation of the cisoid conformer of 2,2′-binaphthyl moiety as observed for the related receptors,8 and concomitant increases of peaks at 322, 292.5, and 281 nm during the course of the addition of Zn(NO3)2 as shown in Fig. S3.† The association constants of 1 with Zn2+ were calculated from the titration data by curve fitting analysis based on a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 binding isotherm and K11 and K12 were determined to be >107 and 2.93 ± 0.04 × 106 mol−1 dm3, respectively. Hamachi et al. reported that K11 and K12 for the complexation of 1,8-bis[(2,2′-dipyridylmethylamino)methyl]anthracene with Zn(NO3)2 in 10 mM HEPES buffer (pH 7.2) were >106 and ∼3 × 105 mol−1 dm3, respectively,9 and the second binding constant was significantly smaller than that of 1. Interestingly, less significant changes on UV-vis spectra of 1 in more acidic condition (20% MeCN–10 mM MES, pH 5.6) were observed as depicted in Fig. S4,† and the second binding constant (K11 > 107 and K12 = 7.25 ± 1.54 × 104 mol−1 dm3) was two orders of magnitude smaller than that in the neutral condition. Receptor 1 showed weak fluorescence due to photoinduced electron transfer from the nitrogen atoms of DPA. Fig. S5† shows fluorescence spectral changes of 1 upon the addition of Zn(NO3)2 in 20% MeCN–MES buffer (pH 5.6) excited at 296 nm, which is one of the isosbestic points of UV-vis titration of 1 with Zn2+. The emissions at 367 and 380 nm were increased upon the addition of Zn2+. In neutral condition (pH 7.2), more drastic fluorescence enhancement at 385 nm was observed upon the addition of Zn2+ (Fig. S6†). The binding constants of 1 in such pHs were calculated from the curve fitting analysis based on the 1
2 binding isotherm and K11 and K12 were determined to be >107 and 2.93 ± 0.04 × 106 mol−1 dm3, respectively. Hamachi et al. reported that K11 and K12 for the complexation of 1,8-bis[(2,2′-dipyridylmethylamino)methyl]anthracene with Zn(NO3)2 in 10 mM HEPES buffer (pH 7.2) were >106 and ∼3 × 105 mol−1 dm3, respectively,9 and the second binding constant was significantly smaller than that of 1. Interestingly, less significant changes on UV-vis spectra of 1 in more acidic condition (20% MeCN–10 mM MES, pH 5.6) were observed as depicted in Fig. S4,† and the second binding constant (K11 > 107 and K12 = 7.25 ± 1.54 × 104 mol−1 dm3) was two orders of magnitude smaller than that in the neutral condition. Receptor 1 showed weak fluorescence due to photoinduced electron transfer from the nitrogen atoms of DPA. Fig. S5† shows fluorescence spectral changes of 1 upon the addition of Zn(NO3)2 in 20% MeCN–MES buffer (pH 5.6) excited at 296 nm, which is one of the isosbestic points of UV-vis titration of 1 with Zn2+. The emissions at 367 and 380 nm were increased upon the addition of Zn2+. In neutral condition (pH 7.2), more drastic fluorescence enhancement at 385 nm was observed upon the addition of Zn2+ (Fig. S6†). The binding constants of 1 in such pHs were calculated from the curve fitting analysis based on the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 binding isotherm as discussed above and the results are collected in Table 1. The binding constants are fairly good agreement with the corresponding values from the UV-vis titrations. 1·2[Zn(NO3)2]·H2O was prepared from 1 with 2 equiv. of Zn(NO3)2·6H2O and the product was confirmed by elemental analysis (see ESI†).
2 binding isotherm as discussed above and the results are collected in Table 1. The binding constants are fairly good agreement with the corresponding values from the UV-vis titrations. 1·2[Zn(NO3)2]·H2O was prepared from 1 with 2 equiv. of Zn(NO3)2·6H2O and the product was confirmed by elemental analysis (see ESI†).
| pH | UV-visa | Fluorescenceb | ||
|---|---|---|---|---|
| K 11/mol−1 dm3 | K 12/mol−1 dm3 | K 11/mol−1 dm3 | K 12/mol−1 dm3 | |
| a Determined by UV-vis spectroscopy. [1] = 2.0 × 10−5 mol dm−3 at 298 K. b Determined by fluorescence spectroscopy. [1] = 1.0 × 10−5 mol dm−3 at 298 K, λex = 296 nm. c 10 mM MES buffer. d 10 mM HEPES buffer. | ||||
| 5.6c | >107 | 7.25 ± 1.54 × 104 | >107 | 3.15 ± 0.05 × 104 |
| 6.8d | >107 | 6.00 ± 0.40 × 105 | ||
| 7.2d | >107 | 2.93 ± 0.04 × 106 | 5.39 ± 1.70 × 106 | 2.43 ± 0.54 × 106 |
Fig. 1A shows pH dependence on UV-vis spectra of 1 in the presence of 2 equiv. of Zn(NO3)2 in 20% MeCN–buffer solution. As the solution pH was increased, the absorbance at 308 nm was decreased with increasing the peak at around 345 nm through isosbestic points indicating existing of two species in the solution. The sigmoidal changes of absorbances at 308 and 345 nm suggested that the pKa of the zinc complex of 1 was estimated to be 6.6 as shown in Fig. 1B. It should be noted that negligible pH dependence on the UV-vis spectra of 1 was observed in the absence of Zn2+ (Fig. S7† and 1B). Kimura and co-worker reported that a bis(Zn2+-cyclen) complex forms a water and hydroxide-bridging dinuclear Zn2+ complex or a μ-hydroxo complex in basic condition and the pKa of the coordinated water molecule of the complex was estimated to be 6.72.10 It can be surmised that the addition of Zn2+ into a solution of 1 in neutral condition provides formation of a water molecule and hydroxide-bridging (1·2Zn2+·H2O·OH−) or μ-OH− (1·2Zn2+·OH−) complex of 1 with two Zn2+ ions. Plausible equilibria of the complexation of 1 with Zn2+ in aqueous solution are shown in Scheme 2. In acidic condition, two DPA units separately coordinate two Zn2+ to form 1·2Zn2+·2H2O. In more basic (or neutral) condition, a coordinated water molecule on Zn2+ is deprotonated to form 1·2Zn2+·H2O·HO− or 1·2Zn2+·HO−, which can be recognized as an anion complex of 1·2Zn2+. The structure of 1·2Zn2+·H2O·HO−, therefore, strongly suggests that 1·2Zn2+ can associate any anions by a similar manner with two Lewis acidic zinc centres.
Anion recognition abilities of 1 were studied by UV-vis and fluorescence spectroscopies with anions as their sodium salts in slightly acidic condition, 20% MeCN–MES buffer (10 mM, pH 5.6) to expect drastic structural changes from the transoid/cisoid equilibrium of 1·2Zn2+ to the predominant cisoid conformation of the 1·2Zn2+·anion quaternary complex. The absorbance of 1 in the presence of 2 equiv. of Zn2+ at around 310 nm was decreased sharply with bathochromic shift to 331 nm upon the addition of pyrophosphate (H2P2O72−) and reached plateau by the addition of 1 equiv. of H2P2O72− through isosbestic points at 296 and 330 nm as shown in Fig. 2. Smaller spectral changes were observed upon the addition of H2PO4− and AcO−, and virtually no changes were measured by the addition of NO3−, ClO4−, and Cl− (Fig. 2B and S10†). A Job's plot of 1·2Zn2+ with H2P2O72− showed a minimum at a mole fraction of 0.5 indicating 1·2Zn2+ associates H2P2O72− for 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio (Fig. S11†).
1 ratio (Fig. S11†).
As mentioned above, 1 showed the fluorescence emissions at 367 and 380 nm in the presence of 2 equiv. Zn2+. The fluorescence emission of 1·2Zn2+ was gradually enhanced and reached plateau upon the addition of 1 equiv. of H2P2O72− in 20% MeCN–MES buffer (10 mM, pH 5.6) as shown in Fig. 3, indicating the strong binding of 1·2Zn2+ with H2P2O72−. However, the addition of other anions, such as AcO−, H2PO4−, NO3−, ClO4−, and Cl−, caused small or no fluorescence changes suggesting weak interaction of 1·2Zn2+ with these anions (Fig. S12†). These results clearly showed that 1·2Zn2+ can be used as a selective fluorescence sensor for biologically important H2P2O72−.
The apparent association constants of 1·2Zn2+ for anions were elucidated by non-linear curve fitting analysis of UV-vis and fluorescence titration data and summarized in Table 2. The association constants of 1·2Zn2+ for H2P2O72− by both UV-vis and fluorescence titrations were larger than 107 mol−1 dm3 clearly indicating strong and selective recognition of H2P2O72− by 1·2Zn2+. The association constants of 1·2Zn2+ for AcO− and H2PO4− were at least two orders of magnitude smaller than that for H2P2O72− by the UV-vis titrations and cannot be determined by fluorescence titrations due to the small spectral changes.
| Anion | K app a/mol−1 dm3 | |
|---|---|---|
| UV-visa | Fluorescenceb | |
| a Determined by UV-vis spectroscopy. [1·2Zn2+] = 2.0 × 10−5 mol dm−3 at 298 K. b Determined by fluorescence spectroscopy. [1·2Zn2+] = 1.0 × 10−5 mol dm−3 at 298 K, λex = 296 nm. c Not determined due to small spectral changes. | ||
| AcO− | 7.47 ± 1.36 × 104 | NDc |
| H2PO4− | 5.77 ± 0.08 × 104 | NDc |
| H2P2O72− | >107 | >107 |
| NO3− | NDc | NDc |
| ClO4− | NDc | NDc |
| Cl− | NDc | NDc |
Competitive titrations of 1·2Zn2+ with H2P2O72− in the presence of excess H2PO4− (5 equiv.), AcO− (5 equiv.), and Cl− (5000 equiv.) in 20% MeCN–MES buffer (10 mM, pH 5.6) were also performed and the results are shown in Fig. S13.† In all cases, the absorbance at 310 nm was indeed decreased upon the addition of H2P2O72−, however, the spectral changes were slightly smaller than that in the absence of these anions. These results suggest that the selectivity of 1·2Zn2+ for H2P2O72− is sufficiently high among these anions.
A plausible mechanism for the fluorescence and UV-vis responses of 1·2Zn2+ upon the addition of anions is shown in Scheme 2. Under the fluorescence titration condition, the distributions of free 1, 1·Zn2+, 1·2Zn2+ are calculated to be 1%, 79%, and 20%, respectively from the titration of 1 with Zn2+ ([1] = 1.0 × 10−5, [Zn2+] = 2.0 × 10−5 mol dm−3, K11 = 107, and K12 = 3.15 × 104 mol−1 dm3 were used for the calculation, as shown in Table 1). The dinuclear complex, 1·2Zn2+ showed high fluorescence intensity, however, the mononuclear one (1·Zn2+) was effectively quenched by PET from the free amino group of the non-coordinated DPA group (Fig. S5c†). Then, the fluorescence of 1 even in the presence of 2 equiv. of Zn2+ was weak due to the predominant formation of 1·Zn2+. Upon the addition of anions, in particular H2P2O72−, 1·2Zn2+·anion was predominantly formed and this species showed high fluorescence intensity due to the suppression of PET by coordinating two Zn2+ in DPA units. In the UV-vis spectroscopy, mononuclear (1·Zn2+·H2O) and dinuclear (1·2Zn2+·2H2O) complexes formed the equilibrated transoid and cisoid conformers. However, 1·2Zn2+·anion should form the cisoid conformer by cooperative binding of anions by two Zn2+ sites, therefore, the peak at around 310 nm of 2,2′-binaphthalene moiety was decreased upon the addition of anions.
Conclusions
In conclusion, we have synthesized a 2,2′-binaphthalene-based receptor 1 bearing di(2-pyridylmethyl)aminomethyl subunits at 8- and 8′-positions. The receptor 1 formed dinuclear complex (1·2Zn2+) with Zn2+ in aqueous solution. The complex can selectively recognize biologically important pyrophosphate and both UV-vis and fluorescence spectral changes of 1 in the presence of 2 equiv. of Zn2+ were observed during the complexation even in the presence of other competitive anions.Experimental section
General considerations
All reagents used were of analytical grade. Tetrahydrofuran was dried over Na/benzophenone. UV-vis spectra were recorded on a Shimadzu UV-2500PC spectrometer with a thermal regulator (±0.5 °C). NMR spectra were measured on a JEOL ECA-500 (500 MHz) spectrometer. Electron spray ionization mass spectra (ESI-MS) were recorded on an Applied Biosystems/MDS-Sciex API-100 spectrometer. HRMS (FAB) was recorded on a JEOL JMS-SX-102 mass spectrometer. Fluorescence spectra were recorded on a Hitachi F-4500 fluorescence spectrometer. Column chromatography was performed by using Wakogel C-200 from Wako Chemical Co. Melting points were determined with a Yanagimoto MP-J3 micro melting point apparatus and are uncorrected.Synthesis of 8,8′-bis(di(2-pyridylmethyl)aminomethyl)-2,2′-binaphthalene (1)
Into a mixture of di(2-pyridylmethyl)amine (0.95 g, 4.79 mmol) and triethylamine (0.70 mL, 5.02 mmol) in 15 mL of THF, was added 8,8′-bis(bromomethyl)-2,2′-binaphthalene (0.91 g, 2.05 mmol). The solution was refluxed for 2 h under argon atmosphere. The mixture was evaporated under reduced pressure, then extracted twice with 50 mL of chloroform and 50 mL of aqueous sodium hydrogen carbonate. The combined organic layer was washed with distilled water and dried over anhydrous sodium sulphate. After filtration, the solution was evaporated under reduced pressure. The residue was chromatographed on Al2O3 with ethyl acetate as an eluent. The crude product was recrystallized from ethyl acetate–hexane to give pale yellow powder. Yield: 1.04 g, 74%. Mp. 154.9–158.2 °C. 1H NMR (500 MHz, CDCl3): δ 8.55 (s, 2H), 8.45 (d, 4H, J = 4.6 Hz), 7.96 (d, 2H, J = 8.6 Hz), 7.86 (dd, 2H, J1 = 8.6, J2 = 1.7 Hz), 7.83 (d, 2H, J = 8.1 Hz), 7.67 (d, 2H, J = 6.3 Hz), 7.45 (dd, 2H, J1 = 8.1, J2 = 6.3 Hz), 7.44–7.40 (m, 8H), 7.04–7.01 (m, 4H), 4.22 (s, 4H), 3.86 (s, 8H). 13C NMR (126 MHz, CDCl3): δ 159.63, 148.91, 138.74, 136.33, 134.93, 133.03, 132.60, 128.82, 128.21, 127.76, 126.03, 125.40, 123.44, 123.05, 121.90, 60.75, 57.60. Anal. found C, 81.68; H, 6.07; N, 12.28. Calcd for C46H40N6: C, 81.63; H, 5.96; H, 12.42%. FAB-MS (HRMS) calcd for C46H40N6H: m/z 677.3387; found: 677.3364.Determination of the association constants of receptor 1 with anions
The association constants of the receptor with anions were determined by UV-vis and fluorescence titrations. All guest anions are commercially available as sodium salts and were dried under reduced pressure for 1 day prior to use. All titration experiments were carried out with 3 mL of receptor 1 solution in a quartz cell at 25 ± 0.5 °C, and UV-vis and fluorescence spectra were recorded upon the addition of aliquots of the stock solution of appropriate guest anions with a microsyringe. The titration data were analysed with the self-written multi-wavelength curve fitting program on Microsoft Windows 7.Acknowledgements
The authors would like to thank Professor Masaki Yamamura, Tsukuba University for the measurement of HRMS (FAB) of the compound. This work was supported in part by a Grant-in-Aid for Scientific Research (C) from Japan Society for the Promotion of Science and YU-COE (E), Yamagata University.Notes and references
- S. Kubik, C. Reyheller and S. Stüwe, J. Inclusion Phenom. Macrocyclic Chem., 2005, 52, 137–187 CrossRef CAS PubMed.
- J. K. Heinonen, Biological Role of Inorganic Pyrophosphate, Kluwer Academic Publishers, Norwell, 2001 Search PubMed.
- (a) J. F. Callan, A. P. de Silva and D. C. Magri, Tetrahedron, 2005, 61, 8551–8588 CrossRef CAS PubMed; (b) D. Curiel, E. J. Hayes and P. D. Beer, in Topics in Fluorescence Spectroscopy. Advanced Concepts in Fluorescence Sensing Part A: Small Molecule Sensing, ed. C. D. Geddes and J. R. Lakowicz, Springer US, New York, 2005, pp. 59–118 Search PubMed; (c) L. Fabbrizzi, M. Licchelli, G. Rabaioli and A. Taglietti, Coord. Chem. Rev., 2000, 205, 85–108 CrossRef CAS; (d) T. Gunnlaugsson, M. Glynn, G. M. Tocci, P. E. Kruger and F. M. Pfeffer, Coord. Chem. Rev., 2006, 250, 3094–3117 CrossRef CAS PubMed; (e) R. Martínez-Máñez and F. Sancenón, Chem. Rev., 2003, 103, 4419–4476 CrossRef PubMed; (f) R. Martínez-Máñez and F. Sancenón, J. Fluoresc., 2005, 15, 267–285 CrossRef PubMed.
- H. T. Ngo, X. Liu and K. A. Jolliffe, Chem. Soc. Rev., 2012, 41, 4928–4965 RSC.
- (a) D. H. Lee, S. Y. Kim and J.-I. Hong, Angew. Chem., Int. Ed., 2004, 43, 4777–4780 CrossRef CAS PubMed; (b) H. K. Cho, D. H. Lee and J.-I. Hong, Chem. Commun., 2005, 1690–1692 RSC; (c) H. N. Lee, Z. Xu, S. K. Kim, K. M. K. Swamy, Y. Kim, S.-J. Kim and K. Yoon, J. Am. Chem. Soc., 2007, 129, 3828–3829 CrossRef CAS PubMed; (d) H. N. Lee, K. M. K. Swamy, S. K. Kim, J.-Y. Kwon, Y. Kim, S.-J. Kim, Y. J. Yoon and J. Yoon, Org. Lett., 2007, 9, 243–246 CrossRef CAS PubMed; (e) A. Ojida, I. Takashima, T. Kohira, H. Nonaka and I. Hamachi, J. Am. Chem. Soc., 2008, 130, 12095–12101 CrossRef CAS PubMed; (f) G. Su, Z. Liu, Z. Xie, F. Qian, W. He and Z. Guo, Dalton Trans., 2009, 7888–7890 RSC; (g) C. Park and J.-I. Hong, Tetrahedron Lett., 2010, 51, 1960–1962 CrossRef CAS PubMed; (h) H. J. Kim, J. H. Lee and J.-I. Hong, Tetrahedron Lett., 2011, 52, 4944–4946 CrossRef CAS PubMed; (i) P. Das, S. Bhattacharya, S. Mishra and A. Das, Chem. Commun., 2011, 47, 8118–8120 RSC; (j) Y. J. Jang, E. J. Jun, Y. J. Lee, Y. S. Kim, J. S. Kim and J. Yoon, J. Org. Chem., 2005, 70, 9603–9606 CrossRef CAS PubMed; (k) K. Ghosh, A. R. Sarkar, A. Samadder and A. R. Khuda-Bukhsh, Org. Lett., 2012, 14, 4314–4317 CrossRef CAS PubMed; (l) S. Yang, G. Feng and N. H. Williams, Org. Biomol. Chem., 2012, 10, 5606–5612 RSC.
- D. H. Lee, J. H. Im, S. U. Son, Y. K. Chung and J.-I. Hong, J. Am. Chem. Soc., 2003, 125, 7752–7753 CrossRef CAS PubMed.
- J. H. Lee, A. R. Jeong, J.-H. Jung, C.-M. Park and J.-I. Hong, J. Org. Chem., 2011, 76, 417–423 CrossRef CAS PubMed.
- (a) S. Kondo, M. Nagamine and Y. Yano, Tetrahedron Lett., 2003, 44, 8801–8804 CrossRef CAS PubMed; (b) S. Kondo, T. Kinjo and Y. Yano, Bioorg. Med. Chem. Lett., 2004, 14, 1641–1643 CrossRef CAS PubMed; (c) S. Kondo, T. Kinjo and Y. Yano, Tetrahedron Lett., 2005, 46, 3183–3186 CrossRef CAS PubMed; (d) S. Kondo and M. Sato, Tetrahedron, 2006, 62, 4844–4850 CrossRef CAS PubMed; (e) S. Kondo, Supramol. Chem., 2011, 23, 29–36 CrossRef CAS; (f) S. Kondo, M. Nagamine, S. Karasawa, M. Ishihara, M. Unno and Y. Yano, Tetrahedron, 2011, 67, 943–950 CrossRef CAS PubMed; (g) S. Kondo, H. Sonoda, T. Katsu and M. Unno, Sens. Actuators, B, 2011, 160, 684–690 CrossRef CAS PubMed.
- A. Ojida, Y. Mito-oka, K. Sada and I. Hamachi, J. Am. Chem. Soc., 2004, 126, 2454–2463 CrossRef CAS PubMed.
- H. Fujioka, T. Koike, N. Yamada and E. Kimura, Heterocycles, 1996, 42, 775–787 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: 1H and 13C NMR spectra of receptor 1, 1H NMR titration of 1 with Zn(NO3)2·6H2O, preparation of 1·2[Zn(NO3)2]·H2O, UV-vis and fluorescence spectroscopic titrations, and a Job plot. See DOI: 10.1039/c4ra01941e |
| This journal is © The Royal Society of Chemistry 2014 |