Ratiometric fluorescence probes based on a Michael acceptor type of coumarin and their application for the multichannel imaging of in vivo glutathione†
Abstract
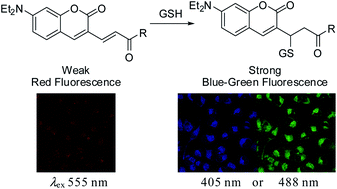
A series of Michael acceptors based on a coumarin moiety, were developed as fluorescent probes for ratiometric detection of in vivo glutathione. The α,β-unsaturated Michael acceptors were transformed into non-conjugated molecules through the Michael addition of biothiols. The resulting UV-vis and fluorescence spectra of the probes revealed characteristic ratiometric responses, which were successfully applied for the multichannel imaging of in vivo glutathione.

 Please wait while we load your content...
Please wait while we load your content...