Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A family of polynuclear cobalt complexes upon employment of an indeno-quinoxaline based oxime ligand†
Angelos B. Canaja,
Lydia E. Nodarakia,
Katarzyna Ślepokurab,
Milosz Siczekb,
Demetrios I. Tzimopoulosc,
Tadeusz Lisb and
Constantinos J. Milios*a
aDepartment of Chemistry, University of Crete, 71003 Herakleion, Greece. E-mail: komil@chemistry.uoc.gr; Tel: +30-2810-545099
bFaculty of Chemistry, University of Wroclaw, Joliot-Curie 14, 50-383 Wroclaw, Poland
cDepartment of Chemistry, Aristotle University of Thessaloniki, 54124, Thessaloniki, Greece
First published on 13th May 2014
Abstract
The reaction of Co(OAc)2·4H2O with LH (LH = 11H-indeno[1,2-b]quinoxalin-11-one oxime) in MeOH in the presence of NEt3 forms the complex [CoIII2CoIIO(OAc)3L3]·0.5MeOH·0.2H2O (1·0.5MeOH·0.2H2O), while repeating the reaction under solvothermal conditions yielded the heptanuclear cluster [CoII7L9 (OH)2(OAc)2.7(MeO)0.3(H2O)]·4.6MeOH·3.3H2O (2·4.6MeOH·3.3H2O). Changing the starting metal salt to Co(ClO4)2·6H2O and upon the reaction with LH in the presence of NEt3 under high temperature and pressure, we managed to isolate the decanuclear cluster [CoII10L14(OH)3.6(MeO)0.4](ClO4)2·8.5MeOH·5.75H2O (3·8.5MeOH·5.75H2O), while under normal bench conditions and upon employment of pivalates in the reaction mixture complex [CoII4L4(piv)4(MeOH)2]·MeOH·H2O (4·MeOH·H2O) was formed. Furthermore, the reaction of Co(ClO4)2·6H2O with LH and aibH (2-amino-isobutyric acid) in the presence of NEt3 in MeOH gave the mononuclear complex [CoIIIL(aib)2]·3H2O (5·3H2O), while upon increasing the metal–ligand ratio cluster [CoIII2CoIIL4(aib)2(OH)2]·7.9MeOH (6·7.9MeOH) was isolated. Finally, repeating the reaction that yielded the mononuclear complex 5·3H2O under solvothermal conditions, gave the octanuclear cluster [CoII8L10(aib)2(MeO)2](ClO4)2·6.8MeOH·7H2O (7·6.8MeOH·7H2O). Variable temperature dc magnetic susceptibility studies for complexes 2, 3, 4 and 7, reveal that all clusters display dominant antiferromagnetic interactions leading to small or diamagnetic ground-states, S.
Introduction
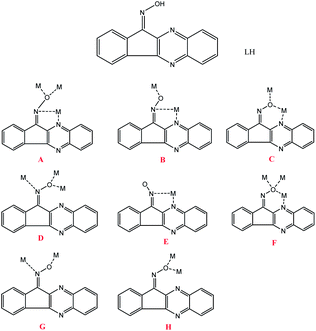
In the last few years metal-oxime coordination chemistry has proven to be a fruitful source for the synthesis and characterization of numerous metallic complexes.1 Although initially triggered by the employment of salicyl- and pyridyl-based oximes in manganese cluster chemistry,2 it later expanded to other 3d, 4f and 3d–4f clusters as well, while a palette of various oxime-based ligands is nowadays utilized for the synthesis of such species.3 In addition, many of these clusters have been found to display interesting magnetic properties, such as single molecule magnetism behaviour (SMM), i.e. they can retain their magnetization once magnetized at very low temperatures in the absence of an external magnetic field.We recently reported the use of a new indeno-quinoxaline based oxime ligand, LH (Scheme 1), in Ni(II) chemistry, which led to the synthesis of five nickel complexes with nuclearities ranging from 3 up to 8.4 We have now expanded our studies in Co(II/III) chemistry, and herein we report the use of this indeno-quinoxaline oxime ligand for the synthesis of an extended family of cobalt complexes.
Experimental
Materials and physical measurements
All manipulations were performed under aerobic conditions using materials as received (reagent grade). Caution! Although we encountered no problems, care should be taken when using the potentially explosive perchlorate ions. LH was synthesized as described in the literature.5 Elemental analyses (C, H, N) were performed by the University of Ioannina microanalysis service. Variable-temperature, solid-state direct current (dc) magnetic susceptibility data down to 5.0 K were collected on a Quantum Design MPMS-XL SQUID magnetometer (University of Crete) equipped with a 7 T dc magnet. Diamagnetic corrections were applied to the observed paramagnetic susceptibilities using Pascal's constants.Syntheses
X-ray crystallography
Diffraction data for complexes 1, 3, 4 and 6 at low temperatures were collected on an Xcalibur R diffractometer with CCD Ruby and for 2, 5 and 7 on a KM4 diffractometer with a CCD Sapphire camera and Mo Kα radiation (λ = 0.71073 Å). The crystal for 3 was slowly cooled from 230 K to 80 K, at which data collection was carried out. All structures were solved by direct methods and refined by full-matrix least-squares techniques on F2 (SHELXL-97).6 Data collection parameters and structure solution and refinement details are listed in Table S1.†Results and discussion
Syntheses
The reaction between Co(OAc)2·4H2O and LH in the presence of base (NEt3) in MeOH yields the trinuclear cluster [CoIII2CoIIO(OAc)3L3]·0.5MeOH·0.2H2O (1·0.5MeOH·0.2H2O) in moderate yield. Given the employment of excess base in the reaction, it is reasonable that all of the LH ligands found in 1 were in their deprotonated monoanionic form, L−. Furthermore, the acetates present in the reaction can also act as a base, thus aiding the deprotonation of the ligand. Two of the three metallic centers were oxidized in the +3 oxidation state, presumably upon oxidation from the atmospheric dioxygen, a commonly observed phenomenon in cobalt chemistry. In order to investigate the effect of high pressure/temperature on the identity of the product, we repeated the same reaction under solvothermal conditions, and we obtained the heptanuclear cluster [CoII7L9(OH)2(OAc)2.7(MeO)0.3(H2O)]·4.6MeOH·3.6H2O (2·4.6MeOH·3.6H2O) according to eqn (1), proving the effect of the solvothermal conditions on increasing the nuclearity of the product:
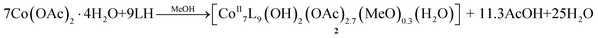 | (1) |
Furthermore, the oxidation of the metallic centers is now suppressed, since in the trinuclear cluster the ratio of CoIII![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CoII was 2
CoII was 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, while in the heptanuclear cluster all cobalt centers are maintained in the 2+ oxidation state, due to the “reducing” environment present within the autoclaves under high temperature/pressure.7 Upon removal of the carboxylates from the reaction mixture, and changing Co(OAc)2·4H2O to Co(ClO4)2·6H2O we managed to isolate the decanuclear cluster [CoII10L14(OH)3.6(MeO)0.4](ClO4)2·8.5MeOH5.75H2O (3·8.5MeOH·5.75H2O) according to eqn (2):
1, while in the heptanuclear cluster all cobalt centers are maintained in the 2+ oxidation state, due to the “reducing” environment present within the autoclaves under high temperature/pressure.7 Upon removal of the carboxylates from the reaction mixture, and changing Co(OAc)2·4H2O to Co(ClO4)2·6H2O we managed to isolate the decanuclear cluster [CoII10L14(OH)3.6(MeO)0.4](ClO4)2·8.5MeOH5.75H2O (3·8.5MeOH·5.75H2O) according to eqn (2):
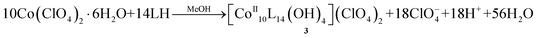 | (2) |
While the reaction forming 3 under normal laboratory conditions did not lead to the formation of any crystalline product, upon addition of pivalates in the reaction mixture we were able to isolate the tetranuclear cluster [CoII4L4(piv)4(MeOH)2]·MeOH·H2O (4·MeOH·H2O), according to eqn (3):
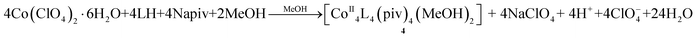 | (3) |
With the identity of cluster 4 established, we checked whether addition of other carboxylates would lead to analogous tetranuclear clusters. Therefore, we employed the artificial amino acid 2-amino-isobutyric acid, aibH, and we managed to characterize the mononuclear complex [CoIIIL(aib)2]·3H2O (5·3H2O), whose identity was not surprising given the chelate coordination mode of the amino acid ligand (vide infra). In order to increase the nuclearity of the cluster, we increased the metal–ligands ratio as a means of “forcing” the cluster to propagate into bigger species due to the lack of sufficient number of chelates. Indeed, upon repeating the same reaction with twice the initial metal salt concentration, we isolated the trinuclear complex [CoIII2CoIIL4(aib)2(OH)2]·7.9MeOH (6·7.9MeOH) in moderate yields. Furthermore, repeating the reaction that yielded the mononuclear complex 5·3H2O under solvothermal conditions, gave the octanuclear cluster [CoII8L10(aib)2(MeO)2] (ClO4)2·6.8MeOH·7H2O (7·6.8MeOH·7H2O) according to eqn (4):
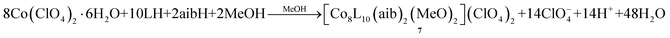 | (4) |
Finally, even though the formation of the complexes containing trivalent CoIII ions, clusters 1, 5 and 6, cannot be “strictly” given by chemical equations since we are not absolutely sure of the oxidizing-reduced species, their formation can be possibly described by the following eqn (5)–(7), assuming that he most likely oxidizing agent responsible for the Co(II) to Co(III) transformation is O2:
 | (5) |
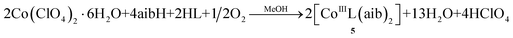 | (6) |
 | (7) |
Description of structures
The molecular structures of complexes 1–7 are presented in Fig. 1–7, while selected interatomic distances and angles are listed in Tables S2–8.† Cluster 1 crystallizes in the trigonal R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) space group. Its structure (Fig. 1) describes an oxo-centered equilateral triangular unit, which consists of three diatomic (–N–O–)oximate bridges, from three deprotonated L− ligands, and three η1:η1:μ acetate ligands. All L− ligands adopt coordination mode B (Scheme 1), while each Co center is in a cis-O4N2 coordination sphere. The formula of the molecule necessitates a [CoIII2CoII] oxidation states' distribution, but since the molecule lies on a special position (on a three-fold axis), and is disordered around it, we cannot assign the oxidation states of the metallic atoms. Similar examples of disordered mixed oxidation states have been previously reported in the literature.8
space group. Its structure (Fig. 1) describes an oxo-centered equilateral triangular unit, which consists of three diatomic (–N–O–)oximate bridges, from three deprotonated L− ligands, and three η1:η1:μ acetate ligands. All L− ligands adopt coordination mode B (Scheme 1), while each Co center is in a cis-O4N2 coordination sphere. The formula of the molecule necessitates a [CoIII2CoII] oxidation states' distribution, but since the molecule lies on a special position (on a three-fold axis), and is disordered around it, we cannot assign the oxidation states of the metallic atoms. Similar examples of disordered mixed oxidation states have been previously reported in the literature.8
 | ||
| Fig. 1 Molecular structure of complex 1. Color code: Co = pink, O = green, N = blue, C = grey. Minor components of acetate and H atoms are omitted for clarity. | ||
Complex 2 crystallizes in the triclinic P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) space group. Its core (Fig. 2) describes an almost planar heptametallic disc. The metallic disk is held together via nine deprotonated monoanionic L− ligands, three acetate and two methoxide ligands. The nine L− ligands found in the structure, adopt four different coordination modes: (i) four are found in mode B (Scheme 1), forming a chelate ring via the oximate and one aromatic N atoms and bridging to a further Co center via the Ooximate atom, (ii) two form a chelate ring via the oximate and one aromatic N atoms and bridging to two further Co centers via the μ-Ooximate atom (mode A), (iii) two are found in a rather unusual mode, bridging three metals via the monoatomic μ-Ooximate bridge and the Noximate atom (mode D), and (iv) one in mode C (Scheme 1) via the Ooximate and Naromatic atoms. Two acetate ligands adopt the usual η1
space group. Its core (Fig. 2) describes an almost planar heptametallic disc. The metallic disk is held together via nine deprotonated monoanionic L− ligands, three acetate and two methoxide ligands. The nine L− ligands found in the structure, adopt four different coordination modes: (i) four are found in mode B (Scheme 1), forming a chelate ring via the oximate and one aromatic N atoms and bridging to a further Co center via the Ooximate atom, (ii) two form a chelate ring via the oximate and one aromatic N atoms and bridging to two further Co centers via the μ-Ooximate atom (mode A), (iii) two are found in a rather unusual mode, bridging three metals via the monoatomic μ-Ooximate bridge and the Noximate atom (mode D), and (iv) one in mode C (Scheme 1) via the Ooximate and Naromatic atoms. Two acetate ligands adopt the usual η1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) η1
η1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) μ mode, while the third one is found terminally bound. Finally, the two hydroxides are found in a μ3 fashion, and in addition a H2O molecule bridges Co2 and Co7 as a monoatomic bridge. All cobalt centers are found in the 2+ oxidation state, as evidenced by their bond distances, as well as BVS calculations (BVS values ranging from 1.76–2.03), and are found in an octahedral geometry adopting cis-O4N2 (Co1, Co2, Co4), cis-O2N4 (Co6, Co3), O5N (Co5) and O6 (Co7) coordination environment.
μ mode, while the third one is found terminally bound. Finally, the two hydroxides are found in a μ3 fashion, and in addition a H2O molecule bridges Co2 and Co7 as a monoatomic bridge. All cobalt centers are found in the 2+ oxidation state, as evidenced by their bond distances, as well as BVS calculations (BVS values ranging from 1.76–2.03), and are found in an octahedral geometry adopting cis-O4N2 (Co1, Co2, Co4), cis-O2N4 (Co6, Co3), O5N (Co5) and O6 (Co7) coordination environment.
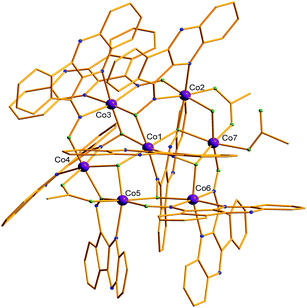 | ||
| Fig. 2 Molecular structure of cluster 2. Color code: same as in Fig. 1. Disorder and H atoms as well as solvent molecules not shown for clarity. | ||
Cluster 3 also crystallizes in the triclinic P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) space group; its metallic core consists of ten edge-sharing [Co3] triangles, forming a slightly bent [Co10] disc (Fig. 3) with dimensions of ∼10 × 6 Å. The ten metallic centers are held in position by fourteen monoanionic L− ligands which adopt six different coordination modes: (i) five are found in mode B, (ii) three in mode F in which the Ooximate forms a six-member chelate ring with the Naromatic and further bridges to two cobalt centers, (iii) two in mode D, (iv) one in mode G, (v) one in mode C, according to which the Ooximate forms a six-member ring with the Naromatic and further bridges to one metallic center, and (vi) two in mode A. In addition, the coordination environment of the metallic centers is completed by the presence of three μ3-OH− and one μ-OH−. Co5, Co8 and Co7 are five-coordinate adopting distorted tetragonal pyramidal for the former two centers and highly distorted trigonal bipyramidal geometry for the remaining center, with τ values of 0.26, 0.30 and 0.62 respectively, while all remaining centers are six-coordinate adopting octahedral geometry.9 Furthermore, the coordination spheres of the metallic centers are O6 (Co1), O5N (Co2, Co9), cis-O2N4 (Co3, Co4, Co6) and fac-O3N3 (Co10) sphere. Finally, all metallic centers are in the 2+ oxidation state, as derived from BVS calculations yielding values in the 1.77–2.04 range.
space group; its metallic core consists of ten edge-sharing [Co3] triangles, forming a slightly bent [Co10] disc (Fig. 3) with dimensions of ∼10 × 6 Å. The ten metallic centers are held in position by fourteen monoanionic L− ligands which adopt six different coordination modes: (i) five are found in mode B, (ii) three in mode F in which the Ooximate forms a six-member chelate ring with the Naromatic and further bridges to two cobalt centers, (iii) two in mode D, (iv) one in mode G, (v) one in mode C, according to which the Ooximate forms a six-member ring with the Naromatic and further bridges to one metallic center, and (vi) two in mode A. In addition, the coordination environment of the metallic centers is completed by the presence of three μ3-OH− and one μ-OH−. Co5, Co8 and Co7 are five-coordinate adopting distorted tetragonal pyramidal for the former two centers and highly distorted trigonal bipyramidal geometry for the remaining center, with τ values of 0.26, 0.30 and 0.62 respectively, while all remaining centers are six-coordinate adopting octahedral geometry.9 Furthermore, the coordination spheres of the metallic centers are O6 (Co1), O5N (Co2, Co9), cis-O2N4 (Co3, Co4, Co6) and fac-O3N3 (Co10) sphere. Finally, all metallic centers are in the 2+ oxidation state, as derived from BVS calculations yielding values in the 1.77–2.04 range.
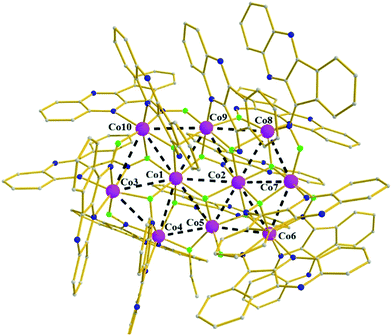 | ||
| Fig. 3 Molecular structure of the cationic part of 3. Color code: same as in Fig. 1. Disorder and H atoms as well as solvent molecules not shown for clarity. | ||
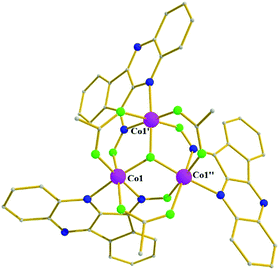
Complex 4 (Fig. 4) crystallizes in the monoclinic space group C2/c in special position of C2 symmetry; its metallic core describes a tetrahedron of four Co2+ ions linked together by (i) four fully deprotonated L− ligands in mode A, forming a distorted [Co4(NO)4]4+ cube comprising alternate single (O) and double (N–O) atom edges, and (ii) two η1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) η1
η1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) μ pivalate ligands. Finally, two terminal pivalate ligands and two methanol molecules complete the coordination environment of the metal centers. The dimensions of the metallic tetrahedron are in the range 3.09–4.43 Å with the shortest distance between Co1 and Co2, and the longest between Co1 and Co1′. All metals are found in the 2+ oxidation state (BVS values: 2.16 for Co1 and 2.17 for Co2, Fig. 4), and are six-coordinate.
μ pivalate ligands. Finally, two terminal pivalate ligands and two methanol molecules complete the coordination environment of the metal centers. The dimensions of the metallic tetrahedron are in the range 3.09–4.43 Å with the shortest distance between Co1 and Co2, and the longest between Co1 and Co1′. All metals are found in the 2+ oxidation state (BVS values: 2.16 for Co1 and 2.17 for Co2, Fig. 4), and are six-coordinate.
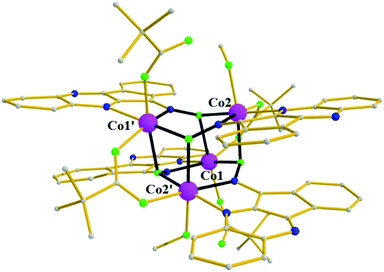 | ||
| Fig. 4 The molecular structure of cluster 4, highlighting its cube-like core. Color code: same as in Fig. 1. Disorder and H atoms not shown for clarity. Symmetry code: ' = −x + 1, y, −z + 1/2. | ||
The mononuclear complex 5 crystallizes in the monoclinic P21/c space group. Its structure (Fig. 5) consists of a central Co3+ ion coordinated by one chelate L− ligand via its oximate and one aromatic N atoms (mode E), and two aib− ligands in a chelate mode, thus forming three five-member chelate rings. The metal ion is found in a cis-O2N4 sphere, while BVS calculations yielded a value of 3.11 for the central metal atom.
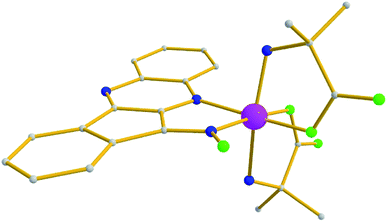 | ||
| Fig. 5 Molecular structure of complex 5. Color code: same as in Fig. 1. Disorder and H atoms as well as solvent molecules not shown for clarity. | ||
Complex 6 crystallizes in the monoclinic space group P21/n. The structure consists of a trimetallic unit with a “V-shape” arrangement of the three metallic centers (Fig. 6), which are held together by two deprotonated ligands, L−, found in mode B, and two μ-OH− groups. Each terminal metallic atom is further coordinated to a deprotonated chelate L− ligand (mode E) and to one chelate aib− ligand. The formula of the complex necessitates a mixed-valent CoIII2CoII oxidation state, and according to BVS calculations the two terminal cobalt atoms are in the 3+ oxidation state (BVS values of 3.15 and 3.22 for Co1 and Co3, respectively), while the central one in the 2+ (BVS value: 2.01). All metallic centers are six-coordinate adopting octahedral geometry, with Co1 and Co3 in a mer-O3N3 coordination sphere, and Co2 in a cis-O4N2 arrangement.
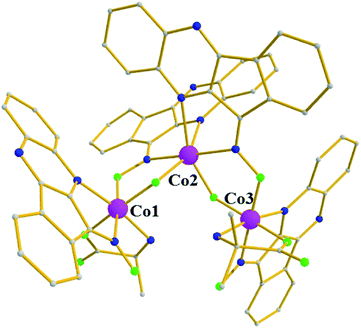 | ||
| Fig. 6 Molecular structure of complex 6. Color code: same as in Fig. 1. Disorder and H atoms not shown for clarity. | ||
Finally, complex 7 crystallizes in the triclinic P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) space group in special position – center of symmetry. Its metallic core (Fig. 7) consists of a planar octametallic disk, in which the eight metallic centers are held in position by ten deprotonated, L−, ligands adopting four different coordination modes; (i) two in mode A, (ii) four in mode B, (iii) two in a monoatomic bridge fashion via the Ooximate, mode H, and (iv) two in mode F. In addition, two deprotonated aib− ligands are present in an η2
space group in special position – center of symmetry. Its metallic core (Fig. 7) consists of a planar octametallic disk, in which the eight metallic centers are held in position by ten deprotonated, L−, ligands adopting four different coordination modes; (i) two in mode A, (ii) four in mode B, (iii) two in a monoatomic bridge fashion via the Ooximate, mode H, and (iv) two in mode F. In addition, two deprotonated aib− ligands are present in an η2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) η1
η1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) η1
η1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) μ3 fashion, while two μ3-OCH3− groups complete the coordination environment of the octametallic disc. All cobalt atoms are six-coordinate, besides Co3 (and its symmetry related Co3′) which is five coordinate adopting tetragonal pyramidal (τ ≈ 0). Finally, all cobalt centers are in the 2+ oxidation state, as evidenced by BVS calculations giving values in the 1.75–2.01 range.
μ3 fashion, while two μ3-OCH3− groups complete the coordination environment of the octametallic disc. All cobalt atoms are six-coordinate, besides Co3 (and its symmetry related Co3′) which is five coordinate adopting tetragonal pyramidal (τ ≈ 0). Finally, all cobalt centers are in the 2+ oxidation state, as evidenced by BVS calculations giving values in the 1.75–2.01 range.
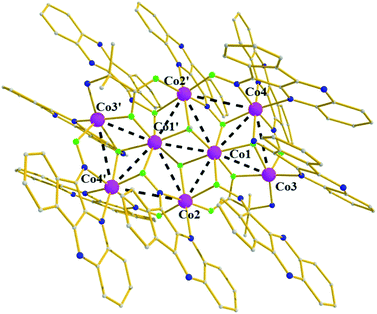 | ||
| Fig. 7 Molecular structure of the cationic part of complex 7. Color code: same as in Fig. 1. H atoms and solvent molecules not shown for clarity. Symmetry code ' = −x + 1, −y + 1, −z + 1. | ||
Magnetochemistry
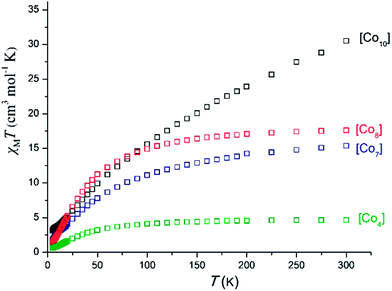 | ||
| Fig. 8 Plot of χMT vs. T for complexes 2 ([Co7]), 3 ([Co10]), 4 ([Co4]) and 7 ([Co8]) under an applied dc field of 1000 G. | ||
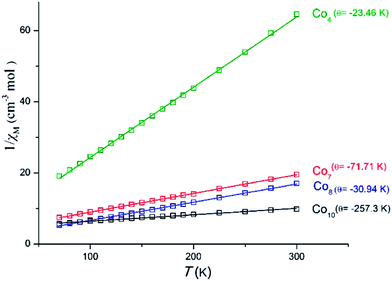
For complex 2 ([Co7]) the room temperature χMT value of 15.39 cm3 K mol−1 corresponds to seven non-interacting CoII ions with S = 3/2 and g = 2.17. Upon cooling the χMT value decreases to reach a minimum value of 1.70 cm3 K mol−1 at 5 K, suggesting the presence of dominant antiferromagnetic interactions. For cluster 4([Co4]) the room temperature χMT value of 4.64 cm3 K mol−1 is very close to the spin-only value of 4.411 cm3 K mol−1 corresponding to two non-interacting CoII ions. Upon cooling, this value remains constant until ∼90 K, before it decreases to reach the minimum value of 0.62 cm3 K mol−1 at 5 K, with this behavior suggesting the presence of dominant, albeit weak, antiferromagnetic interactions. A similar behavior is encountered for cluster 7 ([Co8]); the room temperature χMT value of 17.61 cm3 K mol−1 is very close to the spin-only value of 17.65 cm3 K mol−1 corresponding to eight non-interacting CoII ions. Upon cooling, this value remains constant until ∼120 K, below which it decreases to reach the minimum value of 1.43 cm3 K mol−1 at 5 K.
On the other hand, cluster 3 ([Co10]) displays different behavior than the three complexes already mentioned; the room temperature χMT value of 30.51 cm3 K mol−1 is larger than the expected value for ten non-interacting S = 3/2 ions of 18.7 cm3 K mol−1 (for g = 2), suggesting the presence of significant orbital contribution, as has been previously reported for other [CoII10] clusters.12 Upon cooling the χMT value decreases rapidly to reach the minimum value of 3.12 cm3 K mol−1, suggesting the presence of dominant and strong antiferromagnetic interactions.
The presence of the antiferromagnetic interactions in all complexes was further established by Curie–Weiss analysis of the high temperature (50–300 K) magnetic susceptibility data (Fig. 9), yielding negative θ values for all of them.
Complex 2 joins a small family of twenty six structurally characterized heptanuclear clusters (Table 1), in various oxidation states, while complexes 7 and 3 join a family of structurally characterized octanuclear and decanuclear cobalt clusters, respectively, in which the vast majority consists exclusively of divalent metal atoms (Tables 2 and 3).
| Formula | Ox. states | S | θ (K) | Ref. |
|---|---|---|---|---|
| a H2dea = diethanolamine; poap: tritopic pyridine dihydazone ligand; HL1: pyridine-2-ylmethanol; H3L2: tris[3,5-bis(methyl)-benzoic acid]cyclotricatechylene; Hbm = (1H-benzimidazol)-methanol; H3L3 = H2NC (CH2OH)3; Hhdeo = 2-hydroxy-[1,2-di (pyridin-2-yl)]ethane-1-one; (L4)− is the anion of 2-(pyridine-2-yl)pentane-2-ol-4-one; HL5: 2-methoxy-6-[(methylimino)-methyl]phenol; HL6: 6-methyl-2-pyridone; H2L7: 1,1,1-trifluoro-7-hydroxy-4-methyl-5-aza-hept-3-en-2-one; H4bhqe: 1,2-bis(8-hydroxyquinolin-2-yl)ethane-1,2-diol; Heimp: 2-ethoxy-6-(iminomethyl)phenol; H3thme: 1,1,1-tris(hydroxymethyl)ethane; Immp: 2-iminomethyl-6-methoxy-phenolic anion; bzp = 2-benzoyl pyridine; HL8 = 2-iminomethyl-6-methoxyphenol; HL9 = 2-iminophenyl-6-methoxyphenol. | ||||
| [Co7(dea)6(CH3COO)3] | 4Co2+, 3Co3+ | — | — | 13 |
| [Co7(2poap-2H)5] | 3Co2+, 4Co3+ | — | — | 14 |
| [Co7(L1)12Cl2] | 7Co2+ | — | — | 15 |
| [Co7(L2)2(OAc)4O2(DMF)2] | 7Co2+ | — | — | 16 |
| [Co7(bm)12] | 7Co2+ | 21/2 | +1.16 | 17 |
| [Co7(HL3)6(NO3)3(H2O)3] | 4Co2+, 3Co3+ | 2 | — | 18 |
| [Co7O2(O2CCH3)8(NCO)2(HNPEt3)4] | 7Co2+ | — | — | 19 |
| [Co7(hdeo)6(N3)6] | 7Co2+ | 7/2 | +4.89 | 20 |
| [Co7(OH)6(L4)6] | 6Co2+, 1Co3+ | — | — | 21 |
| [Co7(OH)6(L5)6] | 7Co2+ | 7/2 | +1.16 | 22 |
| [Co7(CH3O)6(L5)6] | 7Co2+ | 7/2 | +0.74 | 22 |
| [Co7(N3)6(L5)6] | 7Co2+ | 7/2 | +1.88 | 22 |
| [Co7(L6)12] | 7Co2+ | — | −3.8 | 23 |
| [Co7(L7)6(MeO)6] | 3Co2+, 4Co3+ | 7/2 | — | 24 |
| [Co7(bhqe)3(OH)2(H2O)6] | 7Co2+ | 7/2 | +12 | 25 |
| [Co7(eimp)6(OMe)6] | 7Co2+ | — | −6.46 | 26 |
| [Co7O4(O2CCMe3)9(HO2CCMe3)(H2O)3] | 3Co2+, 4Co3+ | — | — | 27 |
| [Co7(thme)2(O2CCMe3)8Br2] | 6Co2+, 1Co3+ | 3 | — | 28 |
| [Co7(thme)2(O2CMe)10(H2O)4] | 6Co2+, 1Co3+ | — | — | 28 |
| [Co7S6I3(PEt3)4] | 6Co2+, 1Co3+ | — | — | 29 |
| [Co7(immp)6(CH3O)6] | 7Co2+ | — | −1.39 | 30 |
| [Co7(bzp)6(N3)9(CH3O)3] | 7Co2+ | 7/2 | +37.3 | 31 |
| [Co7(OH)6(L8)6] | 7Co2+ | — | — | 32 |
| [Co7(MeOH)2(OH)6(L8)6] | 7Co2+ | — | — | 32 |
| [Co7(L9)6(OH)6(NO3)2] | 6Co2+, 1Co3+ | — | — | 32 |
| [Co7L9(OH)3(OAc)2(H2O)] | 7Co2 | — | −71.71 | t.w |
| Formula | Ox. states | S | θ (K) | Ref. |
|---|---|---|---|---|
| a H2L1: 1,3-bis-(3-oxo-3-(2-pyridyl)-propionyl)-pyridine; HQ: 8-hydroxyquinoline; L2: modified 2,4,4,5,5-pentamethyl-4,5-dihydro-1H-imidazole-(3-oxide)-1-oxyl; L3: 2,2′-biphenol, 2-hpH: 2-hydroxypyridine; Hchp: 6-chloro-2-hydroxypyridine; H3IBT: 4,5-bis(tetrazol-5yl)imidazole; H2L4: 2,6-bis[5-(2-pyridinyl)-1H-pyrazole-3-yl] pyridine; chp = 6-chloro-2-pyridonate; H4TC4A: p-tert-butylthiacalix[4]arene; H4L5: 3-hydroxysalamo; L6: bis-bidentate ligand. | ||||
| [Co8S6(SPh)8] | 4Co2+, 4Co3+ | — | — | 33 |
| [Co8(NPh)9(PPh3)2] | 7Co2+, 1 Co3+ | — | — | 34 |
| [Co8S6(SPh)8] | 4Co2, 4Co3+ | 35 | ||
| [Co8(L1)4(OH)2(H2O)4(NO3)2(bpy)4] | 8Co2+ | — | — | 36 |
| [Co8OQ12] | 8Co2+ | — | −59 | 37 |
| (PPh4)[Co8S6Br2Cl2(PPh3)4] | 1Co1+, 7Co2+ | — | — | 38 |
| [Co8Piv8(OH)6(HPiv)4(H2O)2(L2)2] | 8Co2+ | — | — | 39 |
| [Co8(OMe)2(L3)4(LH)2(2-hp)4(MeCN)4] | 8Co2+ | 4 | +2.75 | 40 |
| [Co8O2(O2CNiPr2)12] | 8Co2+ | — | — | 41 |
| [Co8(MeCO2)8(OMe)16] | 8Co3+ | — | — | 42 |
| [Co8O4(CH3CO2)6(OMe)4]Cl4(OH2)4 | 2Co2+, 6Co3+ | 43 | ||
| [Co8(chp)10(O3PPh)2(NO3)3(Hchp)2] | 8Co2+ | — | — | 44 |
| [Co8(IBT)12]14− | 2Co2+, 6Co3+ | — | — | 45 |
| [Co8(L4)4(OH)4(MeOH)16] | 8Co2+ | 3/2 | — | 46 |
| [Co8Na2(OH)2(chp)2(O2CCPh3)8(HCO2)6(Hchp)2(EtOAc)2] | 8Co2+ | — | — | 47 |
| [Co8(TC4A)2(N3)2(N6H2)2(CH3COO)4] | 8Co2+ | — | −24.8 | 48 |
| [Co8(L5)4(H2O)2X] (X: H2O or EtOH) | 8Co2+ | 0 | −39 | 49 |
| [Co8O2(L6)6] | 8Co2+ | — | — | 50 |
| [Co8O2(OOCCMe3)12] (n: 2 or 3) | 8Co2+ | — | — | 51 and 52 |
| [CoII8L10(aib)2(MeO)2](ClO4)2 | 8Co2 | −30.9 | t.w. | |
| Formula | Ox. st. | S | θ (K) | Ref. |
|---|---|---|---|---|
| a Q: 8-hydroxyquinoline, chp: 2-chloro-6-pyridonate, o-MeBz = 2-methylbenzoate, mhp: 2-methyl-6-pyridonate, Hmhp: 2-methyl-6-hydroxypyridine, p-tBuBz: 4-tertbutylbenzoate, Piv: (CH3)3CCO2−, L2L3: modified molecules of the starting NIT-Me (2,4,4,5,5-pentamethyl-4,5-dihydro-1H-imidazole-3-oxide-1-oxyl) and/or Im-Me (2,4,4,5,5-pentamethyl-4,5-dihydro-1H-imidazole-1-oxyl), Hdpm: dipivaloylmethane, H3tmp: [1,1,1-tris(hydroxymethyl) propane, EtC(CH2OH)3], AdCO2H: adamantyl carboxylic acid, H3cht: cis,cis-1,3,5-cyclohexanetriol, mhp: 6-methyl-2-pyridonate, Hchp: 6-chloro-2-hydroxypyridine, O2CtBu: pivalate, O2CPhtBu: 4-tert-butylbenzoic acid, O2CPh-2-Ph: 2-biphenylcarboxylic acid. | ||||
| [Co10(Q)12(CO3)4] | 10Co2+ | — | −21.1 | 12 |
| [Co10(OH)6(chp)6(o-MeBz)7(n-C3H7OH)5][o-MeBz] | 10Co2+ | — | — | 53 |
| [Co10(OH)6(mhp)6(p-tBuBz)8(Hmhp)2(CH3CN)2] | 10Co2+ | — | — | 53 |
| [Co10(OH)6(mhp)6(O2CPh)7(Hmhp)3Cl(MeCN)] | 10Co2+ | — | — | 54 |
| [Co10(OH)6(mhp)6(O2CCMe3)7Cl(MeCN)3(Hmhp)] | 10Co2+ | — | — | 54 |
| [Co10(OH)4(chp)10(O2CCMe3)6(EtOH)2] | 10Co2+ | — | — | 54 |
| [Co10Piv14(HPiv)2L22L32] | 10Co2+ | — | — | 55 |
| [Co10O(dpm)4(tmp)4(AdCO2)2(AdCO2H)0.5(MeOH)3.5(H2O)1.5] | 10Co2+ | — | — | 12 |
| [Co10O(OH)(dpm)4(cht)2(Hcht)2(AdCO2)3(EtOH)3] | 10Co2+ | — | — | 12 |
| [Co10(OH)6(mhp)6(O2CCPh3)6(Hmhp)3(HCO3)3] | 9Co2+, 1Co3+ | — | — | 56 |
| [Co10(OH)2(chp)14(O3PCH2CH2PO3)] | 10Co2+ | — | — | 57 |
| [Co(OH)(4-NO2-pz)(3,5-Me2-pz)]10 | 10Co3+ | — | — | 58 |
| [Co10(chp)12(O3PPh)2(O2CPh)4(H2O)4] | 10Co2+ | — | — | 59 |
| [Co10(chp)12(O3PPh)2(O2CtBu)4(H2O)4] | 10Co2+ | — | — | 59 |
| [Co10(chp)12(O3PPh)2(O2CPhtBu)4(H2O)4 | 10Co2+ | — | — | 59 |
| [Co10(chp)12(O3PPh)2(O2CPh-2-Ph)4(H2O)4] | 10Co2+ | — | — | 59 |
| [Co10(chp)12(O3PMe)2(O2CPh-2-Ph)4(H2O)4] | 10Co2+ | — | — | 59 |
| [Co10(chp)6(O3PCH2Ph)2(O2CPh)8(F)2(H2O)2] | 10Co2+ | — | — | 59 |
| [Co10(chp)8(O3PCH2Ph)2(O2CPh)8(F)2(MeCN)2] | 10Co2+ | — | — | 59 |
| [Co10(chp)6(O3PCH2Ph)2(O2CtBu)8(F)2(H2O)2] | 10Co2+ | — | — | 59 |
| [Co10(chp)6(O3PMe)2(O2CtBu)8(F)2(MeCN)4] | 10Co2+ | — | — | 59 |
| [Co10(chp)6(O3PEt)2(O2CPh)8(F)2(MeCN)4] | 10Co2+ | — | — | 59 |
| [Co10(chp)6(O3POct)2(O2CPh)8(F)2(MeCN)4] | 10Co2+ | — | — | 59 |
| [Co10(chp)8(Hchp)2(O3PCH2Nap)(O2CPh)7(OH)3] | 10Co2+ | — | — | 59 |
| [Co10L14(OH)3(MeO)](ClO4)2 | 10Co2+ | — | −257 | t.w |
Complexes 2, 3, 4 and 7 were also studied by alternating current ac magnetic susceptibility studies as a means of investigating potential single molecule magnetism behavior, but no out-of-phase signals were observed, thus ruling out this possibility.
Conclusions
In conclusion, we have reported the syntheses, structures and magnetic properties of seven new Co complexes stabilized by an indeno-quinoxaline based oxime ligand, LH. Following our initial results on nickel cluster chemistry, we have now found that this ligand can lead to the synthesis of cobalt clusters, as well. More importantly, the ligand adopts eight different coordination modes leading to clusters with various nuclearities. Studies are underway for the employment of the ligand in 3d–4f and 4f chemistry, and the findings will be reported in the future.Acknowledgements
CJM would like to thank The Excellence Grant (ARISTEIA-2691) for funding. DIT would like to thank the 89439/2012 Grant of the Aristotle University of Thessaloniki.Notes and references
- See for example: P. Chaudhuri, Coord. Chem. Rev., 2003, 243, 143 CrossRef CAS; C. J. Milios, T. C. Stamatatos and S. P. Perlepes, Polyhedron, 2006, 35, 134–194 CrossRef PubMed; A. G. Smith, P. A. Tasker and D. J. White, Coord. Chem. Rev., 2003, 241, 61 CrossRef; M. Viciano-Chumillas, S. Tanase, I. Mutikainen, U. Turpeinen, L. J. de Jongh and J. Reedijk, Inorg. Chem., 2008, 47, 5919 CrossRef PubMed; P. Chaudhuri, M. Hess, E. Rentschler, T. Weyhermüller and U. Flörke, New J. Chem., 1998, 22, 553 RSC; P. Chaudhuri, M. Hess, T. Weyhermüller, E. Bill, H.-J. Haupt and U. Flörke, Inorg. Chem. Commun., 1998, 1, 39 CrossRef; P. Chaudhuri, M. Winter, U. Flörke and H.-J. Haupt, Inorg. Chim. Acta, 1995, 232, 125 CrossRef; S. Ross, T. Weyhermüller, E. Bill, K. Wieghardt and P. Chaudhuri, Inorg. Chem., 2001, 40, 6656 CrossRef PubMed; D. Burdinski, F. Birkelbach, T. Weyhermüller, U. Flörke, H.-J. Haupt, M. Lengen, A. X. Trautwein, E. Bill, K. Wieghardt and P. Chaudhuri, Inorg. Chem., 1998, 37, 1009 CrossRef.
- Representative refs and refs therein: C. J. Milios, C. P. Raptopoulou, A. Terzis, F. Lloret, R. Vicente, S. P. Perlepes and A. Escuer, Angew. Chem., Int. Ed., 2004, 43, 210 CrossRef CAS PubMed; T. C. Stamatatos, D. Foguet-Albiol, C. C. Stoumpos, C. P. Raptopoulou, A. Terzis, W. Wernsdorfer, S. P. Perlepes and G. Christou, J. Am. Chem. Soc., 2005, 127, 15380 CrossRef PubMed; C. J. Milios, R. Inglis, A. Vinslava, A. Prescimone, S. Parsons, W. Wernsdorfer, S. P. Perlepes, G. Christou and E. K. Brechin, Chem. Commun., 2007, 2738 RSC; C. J. Milios, R. Inglis, L. F. Jones, A. Prescimone, S. Parsons, W. Wernsdorfer and E. K. Brechin, Dalton Trans., 2009, 2812–2822 RSC; C. J. Milios, S. Piligkos and E. K. Brechin, Dalton Trans., 2008, 1809 RSC.
- See for example: A. Chakravorty, Coord. Chem. Rev., 1974, 13, 1 CrossRef CAS; J. A. Bertrand and P. G. Eller, Prog. Inorg. Chem., 1976, 21, 29 CrossRef; A. G. Smith, P. A. Tasker and D. J. White, Coord. Chem. Rev., 2003, 241, 61 CrossRef; V. Y. Kukushkin, D. Tudela and A. J. L. Pombeiro, Coord. Chem. Rev., 1996, 156, 333 CrossRef; V. Y. Kukushkin and A. J. L. Pombeiro, Coord. Chem. Rev., 1999, 181, 147 CrossRef; A. J. L. Pombeiro and V. Y. Kukushkin, in Comprehensive Coordination Chemistry II, ed. J. A. McCleverty and T. C. Meyer, Elsevier, Amsterdam, 2004, vol. 1, p. 631 CrossRef PubMed; K. F. Konidaris, V. Bekiari, E. Katsoulakou, C. P. Raptopoulou, V. Psycharis, S. P. Perlepes, T. C. Stamatatos and E. M. Zoupa, Inorg. Chim. Acta, 2011, 376, 470 CrossRef PubMed; C. Papatriantafyllopoulou, M. Estrader, C. G. Efthymiou, D. Dermitzaki, K. Gkotsis, A. Terzis, C. Diaz and S. P. Perlepes, Polyhedron, 2009, 28, 1652 CrossRef PubMed; T. C. Stamatatos, E. Katsoulakou, A. Terzis, C. P. Raptopoulou, R. E. P. Winpenny and S. P. Perlepes, Polyhedron, 2009, 28, 1638 CrossRef PubMed; C. G. Efthymiou, C. P. Raptopoulou, A. Terzis, S. P. Perlepes, A. Escuer and C. Papatriantafyllopoulou, Polyhedron, 2010, 29, 627 CrossRef PubMed; C. Papatriantafyllopoulou, G. Aromi, A. J. Tasiopoulos, V. Nastopoulos, C. P. Raptopoulou, S. J. Teat, A. Escuer and S. P. Perlepes, Eur. J. Inorg. Chem., 2007, 2761 CrossRef; E. Moushi, C. G. Efthymiou, S. P. Perlepes and C. Papatriantafyllopoulou, Int. J. Inorg. Chem., 2011, 606271 Search PubMed; C. Papatriantafyllopoulou, L. F. Jones, T. D. Nguyen, N. Matamoros-Salvador, L. Cunha-Silva, F. A. A. Paz, J. Rocha, M. Evangelisti, E. K. Brechin and S. P. Perlepes, Dalton Trans., 2008, 3153 RSC; G. C. Vlahopoulou, T. C. Stamatatos, V. Psycharis, S. P. Perlepes and G. Christou, Dalton Trans., 2009, 3647 Search PubMed; A. Escuer, G. Vlahopoulou, S. P. Perlepes, M. Font-Bardia and T. Calvet, Dalton Trans., 2011, 40, 225 RSC; T. C. Stamatatos, E. Diamantopoulou, C. P. Raptopoulou, V. Psycharis, A. Escuer and S. P. Perlepes, Inorg. Chem., 2007, 46, 2350 CrossRef PubMed; C. Papatriantafyllopoulou, T. C. Stamatatos, C. G. Efthymiou, L. Cunha-Silva, F. A. A. Paz, S. P. Perlepes and G. Christou, Inorg. Chem., 2010, 49, 9743 CrossRef PubMed; A. Escuer, G. Vlahopoulou, S. P. Perlepes and F. A. Mautner, Inorg. Chem., 2011, 50, 2468 CrossRef PubMed; C. Papatriantafyllopoulou, T. C. Stamatatos, W. Wernsdorfer, S. J. Teat, A. J. Tasiopoulos, A. Escuer and S. P. Perlepes, Inorg. Chem., 2010, 49, 10486 CrossRef PubMed; K. F. Konidaris, V. Bekiari, E. Katsoulakou, C. P. Raptopoulou, V. Psycharis, E. Manessi-Zoupa, G. E. Kostakis and S. P. Perlepes, Dalton Trans., 2012, 41, 3797 RSC; K. F. Konidaris, C. D. Polyzou, G. E. Kostakis, A. J. Tasiopoulos, O. Roubeau, S. J. Teat, E. Manessi-Zoupa, A. K. Powell and S. P. Perlepes, Dalton Trans., 2012, 41, 2862 RSC.
- A. B. Canaj, L. E. Nodaraki, A. Philippidis, D. I. Tzimopoulos, E. Fotopoulou, M. Siczek, T. Lis and C. J. Milios, RSC Adv., 2013, 3, 13214 RSC.
- B. D. Pearson, R. A. Mitsch and N. H. Cromwell, J. Org. Chem., 1962, 27, 1674 CrossRef CAS.
- G. M. Sheldrick, Acta Crystallogr., Sect. A: Found. Crystallogr., 2008, 64, 112 CrossRef CAS PubMed.
- R. H. Laye and E. J. L. McInnes, Eur. J. Inorg. Chem., 2004, 2811 CrossRef CAS; J. Spandl, I. Brüdgam and H. Hartl, Angew. Chem., Int. Ed., 2001, 40, 4018 CrossRef.
- X. M. Zhang, Y. Z. Zheng, C. R. Li, W. X. Zhang and X. M. Chen, Cryst. Growth Des., 2007, 7, 980 Search PubMed; T. Nakamoto, M. Hanaya, M. Katada, K. Endo, S. Kitagawa and H. Sano, Inorg. Chem., 1997, 36, 4347 CrossRef CAS PubMed.
- A. W. Addison, T. N. Rao, J. Reedijk, J. van Rijn and G. C. Verschoor, Dalton Trans., 1984, 1349 RSC.
- R. L. Carlin, Magnetochemistry, Springer-Verlag, Berlin, 1986 Search PubMed; O. Kahn, Molecular Magnetism, Wiley-VCH, NewYork, 1993 Search PubMed.
- Representative references and refs therein: F. Klöwer, Y. Lan, J. Nehrkorn, O. Waldmann, C. E. Anson and A. K. Powell, Chem.–Eur. J., 2009, 15, 7413 CrossRef CAS PubMed; E. C. Yang, D. N. Hendrickson, W. Wernsdorfer, M. Nakano, L. N. Zakharov, R. D. Sommer, A. L. Rheingold, M. Ledezma-Gairaud and G. Christou, J. Appl. Phys., 2002, 91, 7382 CrossRef PubMed; Y.-Z. Zhang, W. Wernsdorfer, F. Pan, Z.-M. Wang and S. Gao, Chem. Commun., 2006, 3302 RSC; O. Waldmann, M. Ruben, U. Ziener, P. Müller and J. M. Lehn, Inorg. Chem., 2006, 45, 6535 CrossRef PubMed; H. Andres, J. M. Clemente-Juan, M. Aebersold, J. M. Güdel, E. Coronado, H. Buettner, G. Kearly, J. Melero and R. Burriel, J. Am. Chem. Soc., 1999, 121, 10028 CrossRef; A. V. Palii, B. S. Tsukerblat, E. Coronado, J. M. Clement-Juan and J. J. Borras-Almenar, Inorg. Chem., 2003, 42, 2455 CrossRef PubMed; L. Banci, A. Bencini, C. Benelli, D. Gatteschi and C. Zanchini, Struct. Bonding, 1982, 52, 37 CrossRef; R. Boča, Struct. Bonding, 2006, 117, 1 CrossRef; M. Moragues-Canovás, C. E. Talbot-Eeckelaers, L. Catala, F. Lloret, W. Wernsdorfer, E. K. Brechin and T. Mallah, Inorg. Chem., 2006, 45, 7038 CrossRef PubMed; J. M. Clemente-Juan, E. Coronado, A. Gaita-Ariño, C. Giménez-Saiz, H.-U. Güdel, A. Sieber, R. Bircher and H. Mutua, Inorg. Chem., 2005, 44, 3389 CrossRef PubMed; J. M. Clemente-Juan, E. Coronado, A. Forment-Aliaga, J. R. Galan-Mascaros, C. Gimenez-Saiz and C. J. Gomez-Garcia, Inorg. Chem., 2004, 43, 2689 CrossRef PubMed.
- L. Wang, Y. Li, Y. Peng, Z. Liang, J. Yu and R. Xu, Dalton Trans., 2012, 41, 6242 RSC; L. Lisnard, F. Tuna, A. Candini, M. Affronte, R. E. P. Winpenny and E. J. L. McInnes, Angew. Chem., Int. Ed., 2008, 47, 9695 CrossRef CAS PubMed.
- V. Tudor, G. Marin, F. Lloret, V. C. Kravtsov, Y. A. Simonov, M. Julve and M. Andruh, Inorg. Chim. Acta, 2008, 361, 3446 CrossRef CAS PubMed.
- V. Niel, V. A. Milway, L. N. Dawe, H. Grove, S. S. Tandon, T. S. M. Abedin, T. L. Kelly, E. C. Spencer, J. A. K. Howard, J. L. Collins, D. O. Miller and L. K. Thompson, Inorg. Chem., 2008, 47, 176 CrossRef CAS PubMed.
- R. Pattacini, P. Teo, J. Zhang, Y. Lan, A. K. Powell, J. Nehrkorn, O. Waldmann, T. S. A. Hor and P. Braunstein, Dalton Trans., 2011, 40, 10526 RSC.
- T. K. Ronson, H. Nowell, A. Westcott and M. J. Hardie, Chem. Commun., 2011, 47, 176 RSC.
- S. H. Zhang, L. F. Ma, H. H. Zou, Y. G. Wang, H. Liang and M. H. Zeng, Dalton Trans., 2011, 40, 11402 RSC.
- A. Ferguson, A. Parkin, J. Sanchez-Benitez, K. Kamenev, W. Wernsdorfer and M. Murrie, Chem. Commun., 2007, 3473 RSC.
- H. Ackermann, R. Leo, W. Massa and K. Dehnicke, Z. Anorg. Allg. Chem., 2000, 626, 608 CrossRef CAS.
- X. T. Wang, B. W. Wang, Z. M. Wang, W. Zhang and S. Gao, Inorg. Chim. Acta, 2008, 361, 3895 CrossRef CAS PubMed.
- A. A. Kitos, C. G. Efthymiou, C. Papatriantafyllopoulou, V. Nastopoulos, A. J. Tasiopoulos, M. J. Manos, W. Wernsdorfer, G. Christou and S. P. Perlepes, Polyhedron, 2011, 30, 2987 CrossRef CAS PubMed.
- Y. L. Zhou, M. H. Zeng, L. Q. Wei, B. W. Li and M. Kurmoo, Chem. Mater., 2010, 22, 4295 CrossRef CAS.
- A. A. Sidorov, M. E. Nikiforova, E. V. Pakhmutova, G. G. Aleksandrov, V. N. Ikorskii, V. M. Novotortsev, I. L. Eremenko and I. I. Moiseev, Izv. Akad. Nauk SSSR, Ser. Khim. (Russ.) (Russ. Chem. Bull.), 2006, 1851 Search PubMed.
- L. F. Chibotaru, L. Ungur, C. Aronica, H. Elmoll, G. Pilet and D. Luneau, J. Am. Chem. Soc., 2008, 130, 12445 CrossRef CAS PubMed.
- Q. Chen, M. H. Zeng, Y. L. Zhou, H. H. Zou and M. Kurmoo, Chem. Mater., 2010, 22, 2114 CrossRef CAS.
- L. Q. Wei, B. W. Li, S. Hua and M. H. Zeng, CrystEngComm, 2011, 13, 510 RSC.
- G. Aromi, A. S. Batsanov, P. Christian, M. Helliwell, A. Parkin, S. Parsons, A. A. Smith, G. A. Timco and R. E. P. Winpenny, Chem.–Eur. J., 2003, 9, 5142 CrossRef CAS PubMed.
- M. Moragues-Canovas, C. E. Talbot-Eeckelaers, L. Catala, F. Lloret, W. Wernsdorfer, E. K. Brechin and T. Mallah, Inorg. Chem., 2006, 45, 7038 CrossRef CAS PubMed.
- F. Cecconi, C. A. Ghilardi, S. Midollini and A. Orlandini, Inorg. Chim. Acta, 1991, 184, 141 CrossRef CAS.
- S. H. Zhang, Y. Song, H. Liang and M. H. Zeng, CrystEngComm, 2009, 11, 865 RSC.
- Y. Z. Zhang, W. Wernsdorfer, F. Pan, Z. M. Wang and S. Gao, Chem. Commun., 2006, 3302 RSC.
- S. T. Meally, C. McDonald, P. Kealy, S. M. Taylor, E. K. Brechin and L. F. Jones, Dalton Trans., 2012, 41, 5610 RSC.
- G. Christou, K. S. Hagen and R. H. Holm, J. Am. Chem. Soc., 1982, 104, 1744 CrossRef CAS.
- H. Link and D. Fenske, Z. Anorg. Allg. Chem., 1999, 625, 1878 CrossRef CAS.
- G. Christou, K. S. Hagen, J. K. Bashkin and R. H. Holm, Inorg. Chem., 1985, 24, 1010 CrossRef CAS.
- D. Aguila, L. A. Barrios, O. Roubeau, S. J. Teat and G. Aromi, Chem. Commun., 2011, 47, 707 RSC.
- X. N. Chen, W. Xue, J. B. Lin and X. M. Chen, Chem. Commun., 2010, 46, 246 RSC.
- M. Hong, W. Su, R. Cao, F. Jiang, H. Liu and J. Lu, Inorg. Chim. Acta, 1998, 274, 229 CrossRef CAS.
- E. Fursova, O. Kuznetsova, V. Ovcharenko, G. Romanenko, V. Ikorskii, I. Eremenko and A. Sidorov, Polyhedron, 2007, 26, 2079 CrossRef CAS PubMed.
- N. Berg, S. M. Taylor, A. Prescimone, E. K. Brechin and L. F. Jones, CrystEngComm, 2012, 14, 2732 RSC.
- D. B. Dell'Amico, C. Bradicich, F. Calderazzo, A. Guarini, L. Labella, F. Marchetti and A. Tomei, Inorg. Chem., 2002, 41, 2814 CrossRef CAS PubMed.
- J. K. Beattie, T. W. Hambley, J. A. Klepetko, A. F. Masters and P. Turner, Chem. Commun., 1998, 45 RSC.
- J. K. Beattie, T. W. Hambley, J. A. Klepetko, A. F. Masters and P. Turner, Polyhedron, 1997, 16, 2109 CrossRef CAS.
- S. J. Langley, M. Helliwell, R. Sessoli, P. Rosa, W. Wernsdorfer and R. E. P. Winpenny, Chem. Commun., 2005, 5029 RSC.
- M. Dinca, T. D. Harris, A. T. Iavarone and J. R. Long, J. Mol. Struct., 2008, 890, 139 CrossRef CAS PubMed.
- T. Shiga, T. Matsumoto, M. Noguchi, T. Onuki, N. Hoshino, G. N. Newton, M. Nakano and H. Oshio, Chem.–Asian J., 2009, 4, 1660 CrossRef CAS PubMed.
- C. Cadiou, R. A. Coxall, A. Graham, A. Harrison, M. Helliwell, S. Parsons and R. E. P. Winpenny, Chem. Commun., 2002, 1106 RSC.
- Y. Bi, W. Liao, G. Xu, R. Deng, M. Wang, Z. Wu, S. Gao and H. Zhang, Inorg. Chem., 2010, 49, 7735 CrossRef CAS PubMed.
- S. Akine, W. Dong and T. Nabeshima, Inorg. Chem., 2006, 45, 4677 CrossRef CAS PubMed.
- R. W. Saalfrank, V. Seitz, F. W. Heinemann, C. Gobel and R. Herbst-Irmer, J. Chem. Soc., Dalton Trans., 2001, 599 RSC.
- A. A. Sidorov, I. G. Fomina, M. O. Ponina, G. G. Aleksandrov, S. E. Nefedov, I. L. Eremenko and I. I. Moiseev, Izv. Akad. Nauk SSSR, Ser. Khim. (Russ.) (Russ. Chem. Bull.), 2000, 960 Search PubMed.
- A. A. Sidorov, I. G. Fomina, S. S. Talismanov, G. G. Aleksandrov, V. M. Novotortsev, S. E. Nefedov and I. L. Eremenko, Koord. Khim. (Russ.) (Coord. Chem.), 2001, 27, 584 Search PubMed.
- C. Cadiou, M. Helliwell and R. E. P. Winpenny, C. R. Chim., 2003, 6, 241 CrossRef CAS.
- C. Benelli, A. J. Blake, E. K. Brechin, S. J. Coles, A. Graham, S. G. Harris, S. Meier, A. Parkin, S. Parsons, A. M. Seddon and R. E. P. Winpenny, Chem.–Eur. J., 2000, 6, 883 CrossRef CAS.
- E. Fursova, O. Kuznetsova, V. Ovcharenko, G. Romanenko, V. Ikorskii, I. Eremenko and A. Sidorov, Polyhedron, 2007, 26, 2079 CrossRef CAS PubMed.
- A. Graham, S. Meier, S. Parsons and R. E. P. Winpenny, Chem. Commun., 2000, 811 RSC.
- S. Langley, M. Heliwell, R. Sessoli, S. J. Teat and R. E. P. Winpenny, Inorg. Chem., 2008, 47, 497 CrossRef CAS PubMed.
- H. N. Miras, I. Chakraborty and R. G. Raptis, Chem. Commun., 2010, 46, 2569 RSC.
- S. K. Langley, M. Helliwell, S. J. Teat and R. E. P. Winpenny, Dalton Trans., 2012, 41, 12807 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 989901–989909. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c4ra01914h |
| This journal is © The Royal Society of Chemistry 2014 |