Design of lamellar structured POSS/BPZ polybenzoxazine nanocomposites as a novel class of ultra low-k dielectric materials†
Abstract
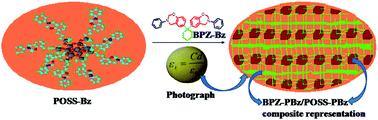
A novel class of lamellar structured polyhedral oligomeric silsesquioxane/bisphenol Z (POSS/BPZ) polybenzoxazine (PBz) nanocomposites was successfully designed by a facile one-step copolymerization technique. The chemical structures of the monomer and resulting polymer were characterized by Fourier transform infrared (FTIR) spectroscopy, 1H, 13C, DEPT-135, 29Si NMR (nuclear magnetic resonance) spectroscopy, X-ray diffraction (XRD), differential scanning calorimetry (DSC) and thermogravimetric analysis (TGA). The desired cross-linked lamellae structural arrangement of POSS/BPZ polybenzoxazine (PBz) nanocomposites was confirmed by transmission electron microscopy (TEM). The BPZ-PBz and POSS-PBz layers were self-assembled by intermolecular hydrogen bonding in such a way as to form the lamellar structure during ring opening polymerization. An advantage of this lamellar structure is that 30% POSS/BPZ polybenzoxazine composite exhibits an ultra low-k value of 1.7 at 1 MHz as well as high thermal stability.

 Please wait while we load your content...
Please wait while we load your content...