High strength biocompatible PEG single-network hydrogels†
Abstract
In this work, PEG-based single-chain hydrogels with extremely high strength were successfully prepared via precise design and control over the molecular topology of the polymeric network. Initially, well-defined PEG macromolecules with uniformly dispersed pendant alkynyl groups (PEGn(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH))m on their main chains were synthesized via amine-epoxy chain extension reaction of α,ω-diepoxy PEG and propargylamine. The subsequent copper(I)-catalyzed azide–alkyne 1,3-dipolar cycloaddition (CuAAC) of (PEGn(C
CH))m on their main chains were synthesized via amine-epoxy chain extension reaction of α,ω-diepoxy PEG and propargylamine. The subsequent copper(I)-catalyzed azide–alkyne 1,3-dipolar cycloaddition (CuAAC) of (PEGn(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH))m and α,ω-diazido PEGn (PEGn(N3)2) gave rise to tough PEG-based hydrogels (Gel-PEGn(N3)2-(PEGn(C
CH))m and α,ω-diazido PEGn (PEGn(N3)2) gave rise to tough PEG-based hydrogels (Gel-PEGn(N3)2-(PEGn(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH))m). The lattice size of the Gel-PEGn(N3)2-(PEGn(C
CH))m). The lattice size of the Gel-PEGn(N3)2-(PEGn(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH))m networks can be tailored by varying chain lengths of PEG repeating segments in (PEGn(C
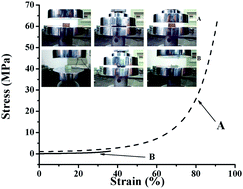
CH))m networks can be tailored by varying chain lengths of PEG repeating segments in (PEGn(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH))m and PEGn(N3)2. Different from traditional PEG hydrogels prepared by CuAAC, such as hydrogels from tetrakis(2-propynyloxymethyl)methane and PEGn(N3)2, the current novel hydrogels possess not only a high mechanical strength up to 62.5 MPa, but are also biodegradable favored by the presence of triethylamine groups in the (PEGn(C
CH))m and PEGn(N3)2. Different from traditional PEG hydrogels prepared by CuAAC, such as hydrogels from tetrakis(2-propynyloxymethyl)methane and PEGn(N3)2, the current novel hydrogels possess not only a high mechanical strength up to 62.5 MPa, but are also biodegradable favored by the presence of triethylamine groups in the (PEGn(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH))m macromolecules. Furthermore, excellent biocompatibility of the Gel-PEGn(N3)2-(PEGn(C
CH))m macromolecules. Furthermore, excellent biocompatibility of the Gel-PEGn(N3)2-(PEGn(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH))m was demonstrated according to the in vitro cytotoxicity assay. Hence, it can be ascertained that Gel-PEGn(N3)2-(PEGn(C
CH))m was demonstrated according to the in vitro cytotoxicity assay. Hence, it can be ascertained that Gel-PEGn(N3)2-(PEGn(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH))m has promising potential for artificial medical devices or scaffolding materials for regenerative medicine.
CH))m has promising potential for artificial medical devices or scaffolding materials for regenerative medicine.

 Please wait while we load your content...
Please wait while we load your content...