Double-walled TiO2 nanotubes prepared with NH4BF4 based electrolyte and their photoelectrochemical performance†
Abstract
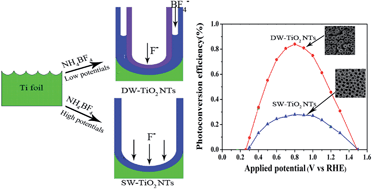
Highly ordered anodic single-walled TiO2 nanotubes (SW-TiO2 NTs) and double-walled TiO2 nanotubes (DW-TiO2 NTs) are prepared in the unique NH4BF4 based electrolyte. The formation of SW-TiO2 NTs and DW-TiO2 NTs can be simply tuned by the voltages. The DW-TiO2 NTs show higher photoelectrochemical performance than the SW-TiO2 NTs.

 Please wait while we load your content...
Please wait while we load your content...