Room temperature acetalization of glycerol to cyclic acetals over anchored silicotungstates under solvent free conditions
Nilesh Narkhede and
Anjali Patel*
Polyoxometalate and Catalysis Laboratory, Department of Chemistry, Faculty of Science, The M. S. University of Baroda, Vadodara, 390002, India. E-mail: aupatel_chem@yahoo.com; Tel: +91-265-2795552
First published on 15th April 2014
Abstract
Heterogeneous catalysts comprising of parent Keggin type silicotungstate as well as monolacunary silicotungstate anchored to MCM-41 were synthesized and characterized by several physicochemical methods. A solvent free green route towards valorisation of glycerol via acetalization with benzaldehyde has been proposed. Both the catalysts showed very good activity as well as selectivity towards dioxolane derivatives within a short reaction time and at room temperature. The tuning of the acidity of the parent silicotungstate leads to an increase in the selectivity towards 1,3-dioxolane. The catalysts were also recycled up to four times without any significant loss in the conversion. The excellent performance of these mesoporous catalysts is attributed to their combination of acidity, wide pores and large specific surface area.
1. Introduction
Glycerol is a side product (10 wt%) of biodiesel synthesis leading to an increase of crude glycerol in the market. Production of value-added products from glycerol is an attractive substitute for the consumption of glycerol. Some latent uses of glycerol include: hydrogen manufacture,1 glycerol acetate as a fuel additive,2 citric acid production, composite additive, cosmetic bonding agent and to propylene glycol synthesis,3 acrolein,4 ethanol and epichlorhydrin,5 and as anti-freezing agent.6 Since glycerol is a massive biodiesel manufacturing by-product, the development of new openings from waste glycerol to value-added products is of great significance to improve the economic viability of biodiesel synthesis.The different reaction procedures for conversion of glycerol to value-added chemicals including oxidation, dehydration, hydrogenolysis, pyrolysis, etherification, esterification, steam reforming, acetalization, polymerization, oligomerization, and others have been reported.7 Amongst, the acetalization of glycerol is the most important methodology for the synthesis of green and cost-effective bio-additive chemicals from glycerol. Glycerol condense with simple carbonyl compounds to deliver isomeric 1,3-dioxane and 1,3-dioxolane products as novel fine chemical intermediates.
These cyclic acetals are potent precursors for the production of green platform compounds 1,3-propanediol and 1,3-dihydroxyacetone.8 The acetalization reaction is also important for protection of carbonyl groups during the manipulation of multifunctional organic compounds.9 They also have direct applications as fragrances, in lacquer industries, in cosmetics, pharmaceuticals, food and beverage additives, in detergents, and as ignition accelerators and antiknock additives in combustion engines10 and in port wine production.11 Glycerol acetals are often used as basis for surfactants.12
Traditionally, the acetalization of glycerol was carried out by using mineral acids as homogeneous catalysts.5 However, the effluent clearance leads to environmental snags and economical dilemmas. These hitches can be overcome by using heterogeneous catalysts such as alumina,13 aluminosilicates,14 resins,15 transition metal complexes,16–18 and mixed metal oxides.5,19,20
Even though acid catalysis by supported HPAs has been greatly expanded from the viewpoint of their variety of structures and compositions, according to our knowledge, there is only one report on acetalization of glycerol by using heteropoly acids (HPAs).21 Ferreira, et al. have reported acetalization of glycerol with acetone over silica immobilized HPAs. The maximum conversion obtained was 97% at 70 °C in 2 h with glycerol: acetone mole ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6.21 However reports on the reactions involving glycerol, such as dehydration,22–25 esterification26 and dichloropropanol synthesis27 by supported HPAs are available in the art.
6.21 However reports on the reactions involving glycerol, such as dehydration,22–25 esterification26 and dichloropropanol synthesis27 by supported HPAs are available in the art.
We have successfully established the synthesis of parent 12-silicotungstic acid (SiW12) as well as mono lacunary silicotungstate (SiW11) anchored to MCM-41 and characterized by different physicochemical techniques.28,29 Both SiW12 and SIW11 based catalyst showed 90% and 81% conversions of oleic acid esterification under mild conditions. In continuation of our previous efforts to explore wider applicability of these catalysts for acid catalysed organic transformations; green, solvent-free and room temperature conversion of glycerol to cyclic acetals have been carried out via acetalization with various aldehydes. Based on the results the catalyst activity has been correlated with the structural features and acidity of the catalysts and the possible mechanism has also been proposed.
2. Experimental
2.1. Materials
All materials used were of A.R. (analytical) grade. 12-Tungstosilicic acid, benzaldehyde, tetraethylorthosilicate (TEOS), glycerol, sodium tungstate, sodium silicate, acetonitrile, n-butylamine and acetone were purchased from Merck.2.2. Synthesis of the support (MCM-41)
MCM-41 was prepared using previously reported procedure.29 27.1 g surfactant (CTAB) was added to the dilute solution of NaOH (2 M, 3.5 mL NaOH in 480 mL double distilled water) with stirring at room temperature. After the solution became clear, 5 mL TEOS was added drop wise and the gel was aged for 2 h at 60 °C. The resulting material was filtered, washed with distilled water, dried in oven and calcined in air at 550 °C for 5 h.2.3. Synthesis of mono lacunary silicotungstate, Na8SiW11O39·11H2O (SiW11)
0.22 mol, 7.2 g sodium tungstate and 0.02 mol, 0.56 g sodium silicate were dissolved in 150 mL double distilled water at 80 °C. The pH was then adjusted to 4.8 by dilute nitric acid. The volume of the mixture was reduced to half and the resulting solution was filtered to remove unreacted silicates. The lacunary heteropoly anion was separated by liquid–liquid extraction with acetone. The extraction was repeated until the acetone extract showed the absence of nitrate ions. The extracted sodium salt of mono lacunary silicotungstate was dried at room temperature in air. The resulting material was designated as SiW11 (Na = 6%; W = 63.8%; Si = 0.89%).2.4. Synthesis of the catalysts (SiW12/SiW11 anchored to MCM-41)
A series of catalysts containing 10–40% of SiW12 anchored to MCM-41 were synthesized by impregnating an aqueous solution of SiW12 (0.1/10–0.4/40 g mL−1 of double distilled water) with MCM-41 (1 g) and dried at 100 °C for 10 h. The resulting materials were designated as 10% SiW12/MCM-41, 20% SiW12/MCM-41, 30% SiW12/MCM-41 and 40% SiW12/MCM-41, respectively.Similarly, catalysts containing 10–40% of SiW11 anchored to MCM-41 were synthesized by impregnation method. MCM-41 (1 g) was impregnated with an aqueous solution of SiW11 (0.1/10–0.4/40 g mL−1 of double distilled water) and dried at 100 °C for 10 h. The obtained materials were treated with 0.1 N HCl, filtered, washed with double distilled water and dried at 100 °C in order to convert the Na form of the catalyst in to the proton form. The resulting materials were designated as 10% SiW11/MCM-41, 20% SiW11/MCM-41, 30% SiW11/MCM-41 and 40% SiW11/MCM-41, respectively.
2.5. Characterization techniques
A detailed study on the characterization of synthesized materials can be found in our earlier publications.28,29 The main characterization of the catalysts such as FT-Raman 29Si-MAS-NMR, XRD and n-butyl amine acidity are given for readers' convenience. The BET surface area measurements were performed in a Micromeritics ASAP 2010 volumetric static adsorption instrument with N2 adsorption at 77 K. The pore size distributions were calculated by BJH adsorption–desorption method. For FT-IR spectra, samples pressed with dried KBr into discs were recorded by using a Perkin-Elmer spectrometer. The Raman spectra were recorded on a FT-Raman Spectrophotometer Model Bruker FRA 106. The magic-angle spinning (MAS) solid state NMR study was carried out on a BRUKER NMR spectrometer. The 29Si NMR spectra were recorded at 121.49 MHz using a with tetra methyl silane as an external standard.2.6. Determination of acidic strength (potentiometric titration method)
A small quantity (0.1 mL) of 0.05 N, n-butylamine in acetonitrile was added to a suspension of 0.5 g of the catalyst in 50 mL of acetonitrile and the system was stirred at 25 °C. Then, the suspension was potentiometrically titrated against 0.05 N, n-butylamine in acetonitrile. The electrode potential variation was measured with a digital pH meter.The acidity of the catalyst measured by this technique allows us to evaluate the total number of acid sites as well as their acidic strength. In order to interpret the results, it is suggested that the initial electrode potential (Ei) indicates the maximum acid strength of the surface sites and the range where the plateau is reached (meq. g−1 solid) indicates the total number of acid sites.21 The acidic strength of surface sites can be assigned according to the following ranges: very strong site, Ei > 100 mV; strong site, 0 < Ei < 100 mV; weak site, −100 < Ei < 0 mV and very weak site, Ei < −100 mV.
2.7. Reaction procedure
A typical acetalization reaction of glycerol with aldehydes was carried out in a 50 mL two-necked round bottom flask under inert atmosphere. The flask was charged with glycerol (10 mmol), benzaldehyde (12 mmol) and 100 mg of catalyst. The reaction mixture was vigorously stirred at room temperature (28–30 °C) under N2 atmosphere for 60 min. The products were analysed using Shimadzu 2014 GC equipped with RTX-5 capillary column (internal diameter: 0.25 mm, length: 30 m). The products were identified by GC-MS and 1H-NMR of the product mixture.2.8. Leaching test
A leaching of the active species from the support makes the catalyst unattractive, and hence, it is necessary to study the leaching of SiW12/SiW11 from the support. Polyoxometalates can be quantitatively characterized by the heteropoly blue colour, which is observed when it reacted with a mild reducing agent such as ascorbic acid. In the present study, this method was used for determining the leaching of SiW12/SiW11 from the support. One gram of catalyst with 10 mL of conductivity water was refluxed for 24 h. Then, 1 mL of the supernatant solution was treated with 10% ascorbic acid. Development of blue colour was not observed, indicating that there was no leaching. The same procedure was repeated with alcohols and the filtrate of the reaction mixture after completion of reaction in order to check the presence of any leached species. The absence of blue colour indicates no leaching.3. Results and discussion
3.1. Catalyst characterization
The elemental analyses performed on 30% SiW12/MCM-41 (theoretical: W = 19%; Si = 27%; observed: W = 17.9%; Si = 27%) and 30% SiW11/MCM-41 (theoretical: W = 15%; Si = 28%; observed: W = 15.2%; Si = 27.6%) were consistent with theoretical expected values.The BET surface area and pore diameter (BJH method) for both the catalysts are presented in the Table 1. The incorporation of active species inside the channels of MCM-41 leads to decrease in the total surface area of both the catalysts. The overall decrease in surface area of both the catalysts with respect to the support gives the first indication of a chemical interaction between SiW11/SiW12 and MCM-41. However, both surface area and pore diameter of 30% SiW11/MCM-41 are higher than those of 30% SiW12/MCM-41. This may be due to the fact that the removal of W–O unit from the parent SiW12 results in decrease in the size of SiW11 species leading to increase in the available space inside the channels of the support.
| Material | Surface area (m2 g−1) | Pore diameter (nm) |
|---|---|---|
| MCM-41 | 659 | 4.79 |
| 30% SiW12/MCM-41 | 349 | 2.92 |
| 30% SiW11/MCM-41 | 536 | 3.96 |
Raman spectra of both the catalysts are shown in Fig. 1. The Raman spectrum of SiW12 shows bands at 1054, 976, 888, 565, and 208 corresponding to νs(W–Od), νas(W–Od), νas(W–Ob–W), νs(W–Oc–W), and νs(W–Oa), respectively (Fig. 1a) where Oa, Ob, Oc and Od corresponds to the oxygen atoms linked to silicon, to oxygen atoms bridging two tungsten (from two different triads for Ob and from the same triad for Oc) and to the terminal oxygen W![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, respectively.30 The Raman spectrum of 30% SiW12/MCM-41 remains almost the same, confirming the retainment of the Keggin structure (Fig. 1b). The Raman spectrum of SiW11 shows typical bands at 971, 890, 814, 521 and 231 cm−1 corresponding to νs(W–Od), νas(W–Od), νas(W–Ob–W), νs(W–Oc–W), and νs(W–Oa), respectively (Fig. 1c). The presence of these bands confirms the formation of lacunary SiW11 species. The catalyst 30% SiW11/MCM-41 showed Raman bands at 969, 879, 793 and 224 cm−1 with respect to νs(W–Od), νas(W–Od), νas(W–Ob–W) and νs(W–Oa), respectively (Fig. 1d). The presence of these bands confirms the intact SiW11 species in 30% SiW11/MCM-41.
O, respectively.30 The Raman spectrum of 30% SiW12/MCM-41 remains almost the same, confirming the retainment of the Keggin structure (Fig. 1b). The Raman spectrum of SiW11 shows typical bands at 971, 890, 814, 521 and 231 cm−1 corresponding to νs(W–Od), νas(W–Od), νas(W–Ob–W), νs(W–Oc–W), and νs(W–Oa), respectively (Fig. 1c). The presence of these bands confirms the formation of lacunary SiW11 species. The catalyst 30% SiW11/MCM-41 showed Raman bands at 969, 879, 793 and 224 cm−1 with respect to νs(W–Od), νas(W–Od), νas(W–Ob–W) and νs(W–Oa), respectively (Fig. 1d). The presence of these bands confirms the intact SiW11 species in 30% SiW11/MCM-41.
Furthermore, the slight shift in the Raman bands for both the catalysts indicates the chemical interaction of the active species SiW11/SiW12 with the surface silanol groups of MCM-41. The establishment of chemical interaction was further confirmed by means of 29Si MAS NMR.
29Si MAS NMR is the most important method to study chemical environment around the silicon nuclei in mesoporous silica materials. Fig. 2 shows the 29Si MAS NMR spectra of MCM-41, 30% SiW12/MCM-41 and 30% SiW11/MCM-41. The presence of resonance originated from Q2 Si(OSi)2(OX)2, Q3 Si(OSi)3(OH) and Q4 Si(OSi)4 in the catalysts indicates that MCM-41 retains its structure in both the catalysts (Table 2). The spectra of the catalysts are relatively broad and low in intensity when compared to MCM-41. This is due to the strong hydrogen bonding between SiW12/SiW11 and Q2 Si(OSi)2(OH)2 (surface silanol groups) of MCM-41.
| Material | Q2, ppm | Q3, ppm | Q4, ppm |
|---|---|---|---|
| 30% SiW12/MCM-41 | −93 | −103 | −110 |
| 30% SiW11/MCM-41 | −102 | −104.6 | −110.5 |
XRD patterns of MCM-41, 30% SiW12/MCM-41 and 30% SiW11/MCM-41 are shown in Fig. 3. The XRD pattern of the MCM-41 shows a sharp reflection around 2θ = 2° corresponding to (100) plane indicating well-ordered hexagonal structure of MCM-41. The comparison of the XRD patterns of MCM-41 and the catalysts reveals that the mesoporous structure of MCM-41 is rather intact even after anchoring of SiW12/SiW11 species. Further the absence of characteristic peaks of crystalline phase of SiW12 as well as SiW11 in the respective catalysts indicates that the active species are highly dispersed inside the hexagonal channels of MCM-41.
The plots of the electrode potential as a function of meq. amine per g of the catalysts are shown in Fig. 4. It is observed that, both the catalysts contain very strong acid sites. The strength of acidic sites in terms of initial electrode potential is shown in Table 3. It is clear from the Table 3 that the incorporation of species SiW12/SiW11 increases the strength of the acid sites of catalysts to a great extent. It is also interesting to note that almost all values are similar in both the catalysts except the acidic strength. The acidic strength of 30% SiW11/MCM-41 is lower than that of 30% SiW12/MCM-41. The reason being, the acidic character of polyoxometalates is mainly due to the acidic addenda atoms i.e. tungsten in the present case and removal of one tungsten–oxygen unit from the parent SiW12 is expected to decrease the acidity of the SiW11. The obtained value is in good agreement with the expected one.
 | ||
| Fig. 4 Potentiometric titration curves of (a) MCM-41, (b) 30% SiW12/MCM-41 and (c) 30% SiW11/MCM-41. | ||
| Material | Acidic strength, mV | Types of acid sites, meq. g−1 | Total acidic sites, meq. g−1 | |
|---|---|---|---|---|
| Very strong | Strong | |||
| MCM-41 | 48 | — | 2.0 | 2.0 |
| 30% SiW12/MCM-41 | 438 | 0.9 | 2.5 | 3.4 |
| 30% SiW11/MCM-41 | 260 | 0.9 | 2.4 | 3.3 |
In order to confirm the distribution of acidic sites, NH3-TPD acidity measurements were carried out. It is clear from the Table 4 that the support is fairly acidic and both weak and strong acid sites are gradually increased in both the catalysts. Also the distribution of the acid sites is almost similar however; the strength of the strong acid sites is high in 30% SiW12/MCM41.
| Sample | NH3 acidity, mmol g−1 | Strength of acid sites, °C | ||
|---|---|---|---|---|
| Weak | Strong | Weak | Strong | |
| MCM-41 | 0.23 | 0.63 | 190 | 410 |
| 30% SiW11/MCM41 | 0.25 | 0.71 | 210 | 440 |
| 30% SiW12/MCM41 | 0.29 | 0.93 | 225 | 650 |
3.2. Catalytic reaction
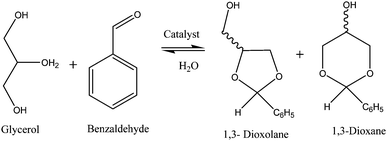
It is well known that lacunary silicotungstates, SiW11 have commendable catalytic activity because of removal of a tungsten–oxygen octahedral moiety from a saturated SiW12 framework leads to an increase and localization of the anionic charge, the resulting lacunary anion becomes highly nucleophilic and reacts easily with electrophilic groups. Hence, the acidic as well as redox properties are altered from the parent saturated unit. This study is focused on catalytic activity of both 30% SiW11/MCM-41 as well as 30% SiW12/MCM-41 for solvent free room temperature acetalization of glycerol (G) with benzaldehyde (B).Acetalization of glycerol with benzaldehyde produces two main products; 1,3-dioxolane and 1,3-dioxane, whose relative formation depends on the acetalization position within the glycerol molecule (Scheme 1). The glycerol acetalization reaction favours the formation of kinetically favoured product, 1,3-dioxolane.
The effect of % loading was studied by carrying out esterification reaction over 10% to 40% loaded catalysts for both the systems (Fig. 5). Low conversions were obtained for low loadings of SiW12/SiW11. The optimum of 91% conversion with 74% 1,3-dioxolane selectivity was obtained by using 30% SiW12/MCM-41 and 85% conversion with 82% 1,3-dioxolane selectivity was obtained by using 30% SiW11/MCM-41. The enhanced activity can be assigned to the increase in SiW12/SiW11 content. Further increase in loading beyond 30% loading no significant increase in the conversion was observed. As a result, 30% loading was optimum and considered for the further studies.
The effect of glycerol to benzaldehyde mole ratio was studied by varying ratio from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 1
1 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.3 over both the catalysts (Fig. 6). The glycerol conversion was found to be increasing with increase in the mole ratio. However the selectivity remains almost the same for both the catalysts. The optimum mole ratio of glycerol: benzaldehyde was found to be 1
1.3 over both the catalysts (Fig. 6). The glycerol conversion was found to be increasing with increase in the mole ratio. However the selectivity remains almost the same for both the catalysts. The optimum mole ratio of glycerol: benzaldehyde was found to be 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 for both catalysts.
1.2 for both catalysts.
 | ||
| Fig. 6 Effect of mole ratio on acetalization of glycerol over (a) 30% SiW12/MCM-41 and (b) 30% SiW11/MCM-41. Reaction conditions: time: 60 min; temperature: 30 °C; catalyst amount: 100 mg. | ||
The effect of the amount of catalysts on glycerol conversion was studied by varying catalyst amount in the range 50–150 mg. As shown in Fig. 7, initial increase in the catalyst concentration increases the conversion of glycerol with almost similar selectivity to dioxolane and reaches saturation conversion at 100 mg of the catalyst amount. Similar trend was observed for both the catalysts. The increase in the conversion can be attributed to an increase in the number of available catalytically active sites. Hence, 100 mg of the catalyst was considered to be optimum for the maximum conversion.
 | ||
| Fig. 7 Effect of catalyst amount on acetalization of glycerol over (a) 30% SiW12/MCM-41 and (b) 30% SiW11/MCM-41. Reaction conditions: mole ratio G/B: 1/1.2; time: 60 min; temperature: 30 °C. | ||
In order to examine the variation of glycerol conversion and products selectivity with time, we have studied acetalization of glycerol at different time intervals (reaction time varied from 15 to 75 min). The conversion of glycerol was increased with increase in the reaction time. It was observed from the Fig. 8 that the kinetically favoured product dioxolane was formed initially as major product and with increase in time the selectivity towards thermodynamically more stable product, dioxane increases slowly. At 60 minutes of the time 91% conversion with 74% selectivity to dioxolane was observed for 30% SiW12/MCM-41 and 85% conversion of glycerol with 82% selectivity to dioxolane was observed for 30% SiW11/MCM-41.
The optimized conditions for maximum conversion (91% for 30% SiW11/MCM-41 and 85% for 30% SiW11/MCM-41) are: mole ratio G/B: 1/1.2; time: 60 min; temperature: 30 °C; catalyst amount: 100 mg. By looking at the industrial importance of the dioxolane derivative, 30% SiW11/MCM-41 will be the choice of better catalyst. Further the catalyst 30% SiW11/MCM-41 was explored for acetalization of glycerol with various substituted benzaldehydes.
3.3. Control experiments
The acetalization of glycerol was carried out without catalyst as well as using MCM-41, and active species (SiW12 and SiW11). It is clear from the Table 5 that the support MCM-41 is not much active towards the acetalization and the activity of the species SiW11/SiW12 has been retained in the catalysts. The activity of 30% SiW12/MCM-41 is higher than 30% SiW11/MCM-41. The results are quite consistent with the acidity of both the catalyst. However the selectivity for 1,3-dioxolane is lower in the case of 30% SiW12/MCM-41 as high acidic strength causes ring transformation to 1,3-dioxane through benzyl cation.| Catalyst | % Conv. | % Sel.b | TON |
|---|---|---|---|
| a Reaction conditions: mole ratio G/B: 1/1.2; time: 60 min; temperature: 30 °C; catalyst amount: *23 mg/**100 mg. TON was calculated from the formula, TON = moles of product/moles of catalyst.b Dioxolane selectivity. | |||
| No catalyst | 15 | 90 | — |
| MCM-41** | 28 | 85 | — |
| SiW11* | 90 | 70 | 1048 |
| 30% SiW11/MCM-41** | 85 | 82 | 989 |
| SiW12* | 95 | 65 | 1188 |
| 30% SiW12/MCM-41** | 91 | 74 | 1139 |
3.4. Heterogeneity test
For the rigorous proof of heterogeneity, a test was carried out by filtering catalyst (30% SiW11/MCM-41) from the reaction mixture after 20 min and allowed the filtrate to react up to 1 h. The reaction mixture of 20 min and filtrate was analysed by gas chromatogram. Both 20 min and final 60 min samples showed same conversion of 23%. It has been reported by Sheldon et al. that there are three categories for a catalyst to behave as a true heterogeneous catalyst in context of leaching of metal from the support (a) the metal leaches but is not active homogeneous catalyst, (b) metal leaches to form an active homogeneous catalyst and (c) the metal does not leach is a true heterogeneous catalyst. The results indicate that the present catalyst fall into category C.31 On the basis of these results, it can be concluded that there is no any leaching of the SiW11 from the support and the present catalysts are truly heterogeneous in nature.3.5. Recycling study
The catalysts were recycled for four times in order to test their activity in successive runs. The catalysts were separated from the reaction mixture by simple centrifugation, washed with 5 mL methanol and then with 5 mL double distilled water, dried at 100 °C in an oven for 10 h and the recovered catalysts were charged for the further runs. The conversion of glycerol observed for four successive runs over both the catalysts are: 85%, 84%, 85% and 82% for 30% SiW11/MCM-41 and 91%, 89%, 88% and 89% for 30% SiW12/MCM-41. Hence, results suggest that catalysts can be reused up to four cycles without significant loss in the conversion.Further the recycled catalysts were characterized by FT-IR analysis and BET surface area in order to see any structural change. The FT-IR spectra of recycled 30% SiW12/MCM-41 showed the retention of typical bands for SiW12, at 979 cm−1 and 923 cm−1 corresponding to W![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Od and Si–Oa symmetric stretching, respectively. The FT-IR spectra of the used catalyst 30% SiW11/MCM-41 (Fig. 9) shows retention of bands at 960 cm−1 (W
Od and Si–Oa symmetric stretching, respectively. The FT-IR spectra of the used catalyst 30% SiW11/MCM-41 (Fig. 9) shows retention of bands at 960 cm−1 (W![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Od), 900 cm−1 (Si–Oa) suggesting that the structure of SiW11 in regenerated catalyst is intact. The BET surface area values of the recycled catalysts (520 for 30% SiW11/MCM-41 and 332 for 30% SiW12/MCM-41) are comparable with the fresh ones (536 for 30% SiW11/MCM-41 and 349 for 30% SiW12/MCM-41).
Od), 900 cm−1 (Si–Oa) suggesting that the structure of SiW11 in regenerated catalyst is intact. The BET surface area values of the recycled catalysts (520 for 30% SiW11/MCM-41 and 332 for 30% SiW12/MCM-41) are comparable with the fresh ones (536 for 30% SiW11/MCM-41 and 349 for 30% SiW12/MCM-41).
3.6. Comparison with the reported catalysts
It is observed from the Table 6 that all the reported catalysts operate at higher temperatures, longer reaction times, low conversion and use of toxic solvents.5,16,32 In contrast our catalyst prefers solvent free condition, requires very short time of 60 min and most importantly both conversion and selectivity are very high. The reaction occurred under solvent-free condition and offered several advantages, such as environmental compatibility, simplifying workup, formation of cleaner products, reduction of byproducts.| Catalyst | Reaction conditionsa | % Conv. | % Sel.b | Ref. |
|---|---|---|---|---|
| a Reaction conditions = amount of catalyst (mg): ratio of G/B: reaction temperature °C: reaction time (minutes).b Dioxolane selectivity. | ||||
| RHABIm–HSO4 | 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1/2 1/2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 120 120![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 120 120 |
61 | 82 | 32 |
| MoOx/TiO2–ZrO2 | 5 wt%![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1/1 1/1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 30 |
54 | 48 | 16 |
| MoO3/SiO2 | 10 wt%![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1/1 1/1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 480 480 |
72 | 40 | 5 |
| 30% SiW12/MCM-41 | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1/1.2 1/1.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60 60 |
91 | 74 | This work |
| 30% SiW11/MCM-41 | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1/1.2 1/1.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60 60 |
85 | 82 | This work |
3.7. The acetalization of glycerol with various aldehydes
The catalyst 30% SiW11/MCM-41 was further explored for carrying out reaction using various substituted aldehydes (Table 7). Acetalization of glycerol with benzaldehyde produced 85% conversion with 82% selectivity for dioxolane. It was found that all substituted compounds exhibit relatively low glycerol conversion than that of benzaldehyde indicating the influence of steric hindrance caused by the presence of substituents. All over high conversions and selectivities reveal the potential of the present catalyst for the acetalization of glycerol even with the substituted benzaldehyde compounds.| Aldehyde | % Conv. | % Sel.b |
|---|---|---|
a Reaction conditions = amount of catalyst (100 mg of 30% SiW11/MCM-41): ratio of G/B (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2): reaction temperature °C (30 °C): reaction time (60 min).b Dioxolane selectivity. 1.2): reaction temperature °C (30 °C): reaction time (60 min).b Dioxolane selectivity. |
||
| Benzaldehyde | 85 | 82 |
| 4-Bromobenzaldehyde | 72 | 65 |
| 4-Methoxybenzaldehyde | 75 | 55 |
| 4-Hydroxybenzaldehyde | 69 | 62 |
3.8. Proposed mechanism for acetalization
The first step is the coordination and activation of the carbonyl group of the benzaldehyde. Then, the carbon atom of the carbonyl group can be attacked by the primary alcoholic group of glycerol followed by the formation of a bond between the carbonyl oxygen atom and the β-carbon of the glycerol. Finally, the dehydration process leads to the formation of dioxolane.There exists equilibrium between dioxolane and dioxane in the presence of strong acid sites via key intermediate benzyl cation. The catalyst 30% SiW12/MCM-41 possesses very strong acid sites than 30% SiW11/MCM-41. The strength of the acid sites leads to the ring transformation in the case of 30% SiW12/MCM-41. Hence, relatively low selectivity was observed for 30% SiW12/MCM-41.
4. Conclusions
The present catalytic systems show very high conversion and selectivity for acetalization of glycerol with benzaldehyde. Very short reaction time, room temperature and solvent free conditions make the process environmentally benign. Tuning acidic strength of the silicotungstate, we have achieved very high conversion (85%) and selectivity (82%). The reusability of the catalysts makes them ideal choice for acetalization of glycerol. The catalyst 30% SiW11/MCM-41 is the better choice amongst them in terms of industrial importance of dioxolane derivative. We have revealed new, highly efficient heterogeneous catalyst for the synthesis of value added product from a renewable feedstock, glycerol thereby opening new perspectives for sustainable valorisation of this side-product of biodiesel production.Acknowledgements
We are thankful to UGC (39-837/2010 (SR)) for the financial support. One of the authors, Mr Nilesh Narkhede, is also thankful to UGC, New Delhi, for the award of Research Fellowship.Notes and references
- A. T. Marshall and R. G. Haverkamp, Int. J. Hydrogen Energy, 2008, 33, 4649–4654 CrossRef CAS PubMed.
- J. A. Melero, R. V. Grieken, G. Morales and M. Paniagua, Energy Fuels, 2007, 21, 1782–1791 CrossRef CAS.
- A. Nemeth and B. Sevella, Appl. Biochem. Biotechnol., 2007, 144, 47–58 CrossRef PubMed.
- L. Ott, M. Bicker and H. Vogel, Green Chem., 2006, 8, 214–220 RSC.
- S. B. Umbarkar, T. V. Kotbagi, A. V. Biradar, R. Pasricha, J. Chanale and M. K. Dongare, et al., J. Mol. Catal. A: Chem., 2009, 310, 150–158 CrossRef CAS PubMed.
- P. H. R. Silva, V. L. C. Gonsalves and C. J. A. Mota, Bioresour. Technol., 2010, 101, 6225–6229 CrossRef CAS PubMed.
- C.-H. Zhou, J. N. Beltramini, Y.-X. Fan and G. Q. Lu, Chem. Soc. Rev., 2008, 37, 527–549 RSC.
- A. J. Showler and P. A. Darley, Chem. Rev., 1967, 67, 427–440 CrossRef CAS.
- T. W. Green and P. G. M. Wuts, Protective Groups in Organic Synthesis, Wiley, New York, 2nd edn, 1991, vol. 4, p. 212 Search PubMed.
- M. J. Climent, A. Corma and A. Velty, Appl. Catal., A, 2004, 263, 155–161 CrossRef CAS PubMed.
- A. Cesar, D. S. Ferreira, J. C. Barbe, A. Bertrand and J. Agric, Food Chem., 2002, 50, 2560–2564 CrossRef PubMed.
- M. Pagliaro, R. Ciriminna, H. Kimura, M. Rossi and C. Della Pina, Angew. Chem., Int. Ed., 2007, 46, 4434–4439 CrossRef CAS PubMed.
- T. Vu Moc and P. Maitte, Bull. Soc. Chim. Fr., 1975, 9, 2558–2560 Search PubMed.
- M. J. Climent, A. Corma, S. Iborra, M. C. Navarro and J. Primo, J. Catal., 1996, 161, 783–789 CrossRef CAS.
- J. Deutsch, A. Martin and H. Lieske, J. Catal., 2007, 245, 428–433 CrossRef CAS PubMed.
- P. Sudarsanam, B. Mallesham, A. N. Prasad, P. S. Reddy and B. M. Reddy, Fuel Process. Technol., 2013, 106, 539–545 CrossRef CAS PubMed.
- C. Crotti, E. Farnetti and N. Guidolin, Green Chem., 2010, 12, 2225–2231 RSC.
- M. Cataldo, E. Nieddu, R. Gavagnin, F. Pinna and G. Strukul, J. Mol. Catal. A: Chem., 1999, 142, 305–316 CrossRef CAS.
- B. Mallesham, P. Sudarsanam, G. Raju and B. M. Reddy, Green Chem., 2013, 15, 478–489 RSC.
- M. S. Khayoon and B. H. Hameed, Appl. Catal., A, 2013, 465, 191–199 CrossRef PubMed.
- P. Ferreira, I. M. Fonseca, A. M. Ramos, J. Vital and J. E. Castanheiro, Appl. Catal., B, 2010, 98, 94–99 CrossRef CAS PubMed.
- H. Atia, U. Armbruster and A. Martin, J. Catal., 2008, 258, 71–82 CrossRef CAS PubMed.
- S.-H. Chai, H.-P. Wang, Y. Liang and B.-Q. Xu, Green Chem., 2008, 10, 1087–1093 RSC.
- L. Ning, Y. Ding, W. Chen, L. Gong, R. Lin and Y. Lu, Chin. J. Catal., 2008, 29, 212–214 CAS.
- S.-H. Chai, H.-P. Wang, Y. Liang and B.-Q. Xu, Appl. Catal., A, 2009, 353, 213–222 CrossRef CAS PubMed.
- P. Ferreira, I. M. Fonseca, A. M. Ramos, J. Vital and J. E. Castanheiro, Appl. Catal., B, 2009, 91, 416–422 CrossRef CAS PubMed.
- S. H. Lee, D. R. Park and H. Kim, Catal. Commun., 2008, 9, 1920–1923 CrossRef CAS PubMed.
- V. Brahmkhatri and A. Patel, in Environmentally Benign Catalysts Based on Heteropolyacids for Clean Organic Transformations, ed. A. Patel, Springer Inc., New York, 2013, pp. 189–208 Search PubMed.
- A. Patel and N. Narkhede, Catal. Sci. Technol., 2013, 3, 3317–3325 CAS.
- N. Narkhede and A. Patel, Ind. Eng. Chem. Res., 2013, 52, 13637–13644 CrossRef CAS.
- R. A. Sheldon, M. Walau, I. W. C. E. Arends and U. Schuchurdt, Acc. Chem. Res., 1998, 31, 485–493 CrossRef CAS.
- F. Adam, H. E. Hassan and K. Mohammed Hello, J. Taiwan Inst. Chem. Eng., 2012, 43, 619–630 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2014 |