An enzyme-responsive supra-amphiphile constructed by pillar[5]arene/acetylcholine molecular recognition†
Guocan Yu*,
Jie Yang,
Danyu Xia and
Yong Yao
Department of Chemistry, Zhejiang University, Hangzhou 310027, P. R. China. E-mail: guocanyu@zju.edu.cn
First published on 8th April 2014
Abstract
A novel molecular recognition motif between a water-soluble pillar[5]arene (WP5) and acetylcholine is established with an association constant of (5.05 ± 0.13) × 104 M−1. Based on this molecular recognition motif, an enzyme-responsive supra-amphiphile is constructed by introducing an amphiphilic guest (PyCh) that is sensitive to acetylcholinesterase. Furthermore, supramolecular hybrid materials are prepared by introducing gold nanoparticles (AuNPs) into these supramolecular systems, which show enzyme-responsive catalytic abilities for the borohydride reduction of 4-nitroaniline.
Introduction
Supra-amphiphiles1 are amphiphiles that are linked by noncovalent interactions, such as π–π stacking interactions, hydrogen bonding, charge-transfer interactions, and electrostatic interactions.2 Because supra-amphiphiles are synthesized through noncovalent interactions, the need for time-consuming organic synthesis can be greatly reduced. Furthermore, functional supramolecular nanostructures can be easily constructed by introducing building blocks with stimuli-responsive functional moieties into the supra-amphiphiles. Numerous external stimuli such as temperature change, pH-change, redox, and light have been utilized in the construction of stimuli-responsive self-assembly systems.3 Among them, enzyme-responsive self-assembly is especially attractive on account of its good biocompatibility and sensitivity, and therefore displays potential applications in biological materials and drug delivery systems.4 Enzymes play significant roles in a series of biochemical processes and aberrations in the enzyme expression level often cause many diseases. Acetylcholine (ACh), one of many neurotransmitters in the autonomic nervous system, has functions both in peripheral nervous system and central nervous system, and is the only neurotransmitter used in the motor division of the somatic nervous system.5 Damage to the cholinergic (acetylcholine-producing) system in the brain has been shown to be plausibly associated with Alzheimer's disease.5a,b Moreover, in cardiac tissue acetylcholine neurotransmission has an inhibitory effect, lowering heart rate. Consequently, the construction of a supra-amphiphile which can be responsive to cholinesterases, such as acetylcholinesterase (AChE), is of particular interest and importance not only in fundamental research but also in practical application to biotechnology and medicine, because only a limited amount of endeavour has been devoted so far to this research area.Pillar[n]arenes, mainly including pillar[5]arenes6 and pillar[6]arenes,7 are a new kind of macrocyclic hosts, next to crown ethers, cyclodextrins, calixarenes, and cucurbiturils.8,9 Compared with the basket-shaped structure of meta-bridged calixarenes, pillar[n]arenes are linked by methylene (–CH2–) bridges at para-positions of 2,5-dialkoxybenzene rings, forming a unique rigid pillar architecture. The unique symmetrical structure and easy functionalization of pillararenes have afforded them superior properties in host–guest recognition. Pillararenes act as useful platforms for the construction of various interesting supramolecular systems, including liquid crystals,7g cyclic dimers,10 chemosensors,11 supramolecular polymers,12 drug delivery systems,13 transmembrane channels14 and cell glue.15 A series of external stimuli, such as temperature change,16a,d light,16b pH-change16c and redox,16e have been utilized to develop sophisticated pillararene-based supramolecular systems which were employed in various fields. However, enzyme-responsive pillararene-based self-assembly has not been reported up to now.
Considering that the preparation of self-assemblies possessing novel stimuli-responsiveness is extremely important for the potential application in a broad range of fields, such as memory storage, smart supramolecular polymers, drug delivery systems, sensors, protein probes, and functional nanodevices,17 we are interested in the construction of enzyme-responsive pillararene-based supra-amphiphiles to obtain functional supramolecular systems. Herein, we designed and fabricated an enzyme-responsive supra-amphiphile comprised of a water-soluble pillar[5]arene (WP5) and an amphiphilic guest (PyCh) with choline as the hydrophilic part and pyrene derivative as the hydrophobic section (Scheme 1). Guest PyCh itself self-assembled in water to form nanosheets driven by π–π stacking interactions between the pyrenyl groups. Upon addition of WP5, the nanosheets transformed into nanoparticles due to steric hindrance and electrostatic repulsion generated upon insertion of the anionic hosts. Because of the enzyme-responsiveness of PyCh, both the nanosheets formed by PyCh and the nanoparticles formed by the host–guest complex WP5⊃PyCh changed into nanoribbons in the presence of AChE. Considering the existence of trimethylammonium groups on the surfaces of nanosheets and negative carboxylate anions on the surfaces of nanoparticles, these self-assemblies were further employed to prepare supramolecular hybrid materials by fabrication with gold nanoparticles (AuNPs), which were utilized as catalysts for the borohydride reduction of 4-nitroaniline.
Results and discussion
Host–guest interactions molecular recognition between pillar[5]arene and acetylcholine
The host–guest interactions between WP5 and PyCh were firstly studied by 1H NMR spectroscopy by using acetylcholine iodide (M) as a model compound due to the relatively poor solubility of PyCh. Compared with the spectrum of free M (a in Fig. 2a), the resonance peaks related to protons H1, H2, H3 and H4 of M displayed substantial upfield shifts (Δδ = −0.14, −0.31, −0.24 and −0.14 ppm for H1, H2, H3 and H4, respectively) in the presence of an equivalent amount of WP5 (c in Fig. 2a). The reason was that these protons were shielded by the electron-rich cyclic structure upon forming a threaded structure between WP5 and M. Moreover, extensive broadening effects were observed for the peaks corresponding to protons on M due to complexation dynamics.16b On the other hand, the protons on WP5 also exhibited slight chemical shift changes due to the interactions between WP5 and M. The resonance peaks related to protons Hb on the benzene rings and Hc on the methylene bridges shifted downfield (Δδ = 0.04 and −0.03 ppm for Hb and Hc, respectively). From our previous work, we knew that the cavity of pillar[5]arene could only encapsulate four methylenes.6b Therefore, we speculated that the ester and methyl groups were situated in the cavity of WP5, while the methylenes and trimethylammonium group resided on the rim of WP5 due to the electrostatic interactions between the carboxylate anions on the host and the trimethylammonium cation on the guest.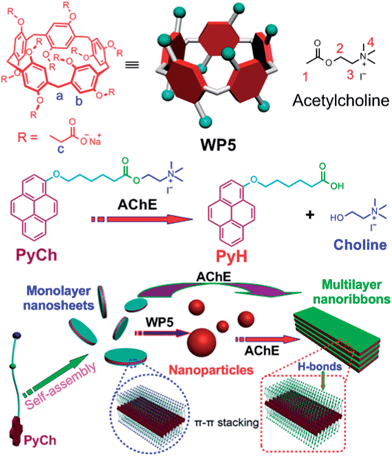 | ||
| Fig. 1 Structural illustration of the building blocks (WP5, PyCh and acetylcholine) and schematic representation of the resulting enzyme-responsive self-assembly. | ||
2D NOESY NMR spectroscopy is a useful tool to study the relative positions of the components in host–guest inclusion complexes. It was used to investigate the complexation between WP5 and M. A nuclear Overhauser effect (NOE) correlation was observed between the signals related to protons H1 on M and protons Hb on WP5 (Fig. S13†), while no NOE was observed between protons H2, H3 and H4 on M and protons on WP5, suggesting that protons H1 were located in the cavity of WP5. To provide further evidence for the host–guest interactions between WP5 and M and to obtain insight into the binding geometry in complex WP5⊃M, molecular modeling was performed (Fig. S15†). The molecular geometry optimization of WP5⊃M shows that the guest is tightly wrapped within WP5. Noticeably, the cationic segment of M is located on the upper side of WP5 to successfully achieve multivalent electrostatic interactions with carboxylate groups on WP5, and the tail of M containing ester and methyl groups locates inside the cavity of WP5. The results obtained from 2D NOESY NMR spectroscopy and molecular modelling were in good agreement with our speculation mentioned above.
Isothermal titration calorimetry (ITC) experiments were performed to provide thermodynamic insight into the inclusion complexation between WP5 and M. From Fig. 2b and Fig. S14,† the Ka value of WP5⊃M was determined to be (5.05 ± 0.13) × 104 M−1 in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complexation. The binding affinity of this host–guest system can be attributed to the cooperativity of multiple electrostatic interactions between the carboxylate anionic groups on the rigid pillar[5]arene receptor platform and the cationic trimethylammonium part and hydrophobic interactions between the alkyl chain and the host. Furthermore, the enthalpy and entropy changes were obtained (ΔH° < 0; TΔS° > 0; |ΔH°| < |TΔS°|), indicating that this complexation was primarily driven by the entropy change with enthalpic assistance (Fig. S14†).
1 complexation. The binding affinity of this host–guest system can be attributed to the cooperativity of multiple electrostatic interactions between the carboxylate anionic groups on the rigid pillar[5]arene receptor platform and the cationic trimethylammonium part and hydrophobic interactions between the alkyl chain and the host. Furthermore, the enthalpy and entropy changes were obtained (ΔH° < 0; TΔS° > 0; |ΔH°| < |TΔS°|), indicating that this complexation was primarily driven by the entropy change with enthalpic assistance (Fig. S14†).
Enzyme-responsive self-assembly of the supra-amphiphile
After the establishment of the pillar[5]arene/acetylcholine recognition motif in water, we applied it to construct an enzyme-responsive supra-amphiphile and studied the controllable self-assembly of this supra-amphiphile. The self-assembly of amphiphilic PyCh in water was investigated first. From Fig. S18,† the critical aggregation concentration (CAC) of PyCh was determined to be 1.25 × 10−6 M using the concentration-dependent conductivity. Transmission electron microscopy (TEM) experiments assisted in the visualization of the self-assembly nanostructure of PyCh. As shown in Fig. 3a, two-dimensional nanosheets were observed clearly with very thin thickness. Fluorescence microscopy (Fig. 3d) and scanning electron microscopy (SEM) were further utilized to confirm the morphology of the self-assemlies formed by PyCh, coinciding with the results obtained from TEM. The thickness of the nanosheets was calculated to be about 5 nm (Fig. 3h and S23c†). Notably, the extended length of PyCh is ∼2.3 nm, close to half the thickness of the nanosheets, indicating a bilayer packing mode of PyCh in the nanosheets (Fig. 1). The packing pattern of PyCh in the nanosheets was studied by UV-vis spectroscopy and X-ray diffraction (XRD). An increase in the concentration caused a blue shift (Fig. S22†), which indicated an H-aggregation form, suggesting that adjacent pyrene aromatic rings underwent considerable overlap through π–π stacking interactions (Fig. 1).16c Moreover, the bilayer structure of the membrane was confirmed by XRD. As shown in Fig. S21a,† the thickness of the bilayer was calculated to be 4.5 nm, close to the length of two PyCh molecules with antiparallel packing and overlapped pyrene rings (Fig. 1),18a in accordance with the results obtained from TEM and SEM images.Interestingly, the critical aggregation concentration of PyCh in the presence of an equivalent amount of WP5 increased to 1.52 × 10−4 M (Fig. S19†). The CAC value of PyCh was enhanced pronouncedly by a factor of ca. 122 due to its host–guest complexation with WP5. Moreover, the resultant self-assemblies changed from nanosheets to nanoparticles with an average diameter of about 250 nm (Fig. 3b). SEM also provided convincing insight into the transformation from nanosheets for PyCh to nanoparticles for WP5⊃PyCh (Fig. 3g–i). Dynamic light scattering (DLS) was further employed to confirm the size of the aggregates formed by WP5⊃PyCh. As shown in Fig. S20,† the main diameter distribution of the aggregates was around 263 nm, which was in harmony with the corresponding TEM and SEM images. It should be pointed out that the diameter of the nano-aggregates measured by DLS was little larger than that obtained in TEM images, attributed to the swelling effect of the spherical structures in water.7d
A mechanism was proposed to explain the morphological transformation from nanosheets for PyCh to nanoparticles for WP5⊃PyCh (Fig. 1). The self-assembled structure of the aggregates, formed by the two distinct bilayers, is determined by the curvature of the membrane.18 For PyCh alone, highly directional π–π stacking interactions between the pyrene aromatic rings are achieved in water, thus leading to the formation of 2D self-assembly in a bilayer structure. The size of trimethylammonium cation group is smaller than the cavity of WP5,6b so the anionic hosts insert into the membrane of the nanosheets and form 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 [2]pseudorotaxanes upon complexation with WP5. Due to the existence of steric hindrance and electrostatic repulsion generated upon the insertion of WP5, the membrane curvature of the nanosheets become higher, resulting in the formation of nanoparticles with a spherical structure.18
1 [2]pseudorotaxanes upon complexation with WP5. Due to the existence of steric hindrance and electrostatic repulsion generated upon the insertion of WP5, the membrane curvature of the nanosheets become higher, resulting in the formation of nanoparticles with a spherical structure.18
PyCh contains an alkanoylcholine that can be hydrolyzed to the corresponding acid (PyH) and choline by cholinesterases (Fig. 1), thereby affecting the morphologies of the self-assemblies. Mass spectral measurements were performed to monitor the enzymatic cleavage of the ester bonds of PyCh. As shown by mass spectra of PyCh at different time points in the presence of AChE, the peak at 418.0 corresponding to [PyCh – I]+ weakened after 5 h (Fig. S25†), and almost disappeared 12 h later (Fig. S26†), in concert with the appearance of the peaks at 105.0 and 332.1 related to choline and PyOH, respectively, indicating that almost all of the ester bonds were cleaved. Interestingly, the self-assembly structures of both PyCh and WP5⊃PyCh underwent dramatic changes as revealed by TEM in the presence of AChE. Nanoribbons several micrometers in length and 200–300 nm in width were observed by culturing the nanoparticles formed by WP5⊃PyCh with AChE at 37 °C. Notably, the coexistence of nanoparticles and nanoribbons was also observed (Fig. 3c and S23a†), demonstrating the gradual transformation from nanoparticles to nanoribbons caused by the hydrolysis of PyCh in the presence of AChE. SEM images provided useful information about the morphological changes of the self-assemblies (Fig. 3j–l). Compared with the nanoparticles, dendritic aggregates with much larger size, several micrometers, were observed after the solution had stood for 2 weeks (Fig. 3j) in the presence of AChE. More exciting, we found that these dendritic superstructures were composed of nanoribbons in the plane-to-plane packing mode (Fig. 3k and l). The thickness of the nanoribbons was ∼20 nm, as observed from a broken multiple-nanoribbon (Fig. 3l and S23d†), indicating that the nanoribbons had a multi-layer structure. Similarly, the nanosheets formed by PyCh alone also changed into nanoribbons in the presence of AChE, as verified by TEM (Fig. S23b†). We also knew that the bilayer structure was retained when the nanosheets transformed into nanoribbons through XRD data (Fig. S21b†). It should be noted that the hydrolysis rate of the host–guest complex WP5⊃PyCh by AChE was much slower than that of free PyCh because there existed a dynamic equilibrium between the complexed and uncomplexed states of PyCh, and AChE attacks only the free species.8r
PyCh both in the nanosheets and in the nanoparticles was hydrolyzed into PyH and choline in the presence of AChE, and the amphiphilic PyH self-assembled in water through π–π stacking interactions. On the other hand, interlayer multiple hydrogen bonds due to the existence of carboxylic acid groups on the surface of the nanoribbons caused the formation of multi-layer aggregates. However, the nanosheets self-assembled from PyCh did not pack together to generate larger aggregates owing to the presence of the cationic groups on the surface and the attendant electrostatic repulsion between the nanostructures. It should be pointed out that there was no interaction between PyH and WP5, so the morphologies of the self-assemblies obtained from hydrolysis of the nanosheets and from the nanoparticles were the same, whether or not WP5 was present.
Preparation of supramolecular hybrid materials and application in borohydride reduction of 4-nitroaniline
With the enzyme-responsive self-assemblies in hand, we explored possible applications of these novel supramolecular systems. Considering the existence of trimethylammonium groups on the surfaces of nanosheets and negative carboxylate anions on the surfaces of nanoparticles, these two self-assemblies could be employed to prepare supramolecular hybrid materials with gold nanoparticles.19 Self-assembled organic aggregates have been demonstrated to be useful in the fabrication of metallized self-assemblies.19b By using the present nanosheets and nanoparticles as the templates, gold nanoparticles were directly loaded onto the surfaces of the self-assemblies at room temperature to form supramolecular hybrid materials (AuNPs@nanosheets and AuNPs@nanoparticles). As shown in TEM images (Fig. 4a–c), we found that the AuNPs adhered to the surface of the self-assemblies with an average diameter of about 6 nm (Fig. S27†). As shown in UV-vis spectra (Fig. S28†), the well-known surface plasmon resonance (SPR) of AuNPs around 520 nm was observed, suggesting the successful preparation of supramolecular hybrid materials. Moreover, Fourier transform IR spectroscopy (Fig. S29†) and energy dispersive spectrometry (EDS) experiments (Fig. S30 and S31†) were conducted to confirm the successful preparation of supramolecular hybrid materials.6jNaturally, these hybrids also displayed excellent enzyme responsivity due to the presence of PyCh. For the hybrids, the diameters of the AuNPs became larger and their shapes became smoother and rounder by treatment with AChE due to Ostwald ripening (Fig. 4e and f),6j because the total surface area can be minimized by the formation of spherical particles, and larger particles are more energetically favored than smaller particles. Furthermore, we applied TEM to characterize the aggregation process of AuNPs at the nanoscale before and after aggregation induced by AChE. A greater degree of aggregation was observed for the AuNPs corresponding to AuNPs@nanosheets after hydrolysis by AChE (Fig. 4f). The reasons were as follows: the choline was attached to the surface of AuNPs when PyCh in AuNPs@nanosheets hydrolyzed into PyH and choline, resulting in the growth of AuNPs into larger aggregates (Fig. 4b). However, for the AuNPs@nanoparticles, the choline derived from PyCh formed a stable host–guest complex with WP5. Owing to the presence of carboxylate anionic groups on the macrocyclic ring, the host–guest complex WP5⊃choline can be considered as a cluster of carboxylate groups and sodium, providing a shell of anions and cations around the gold nanoparticles, thus stabilizing them in aqueous solution. Therefore, relatively smaller changes in the size of AuNPs occurred for AuNPs@nanoparticles after the hydrolysis of PyCh (Fig. 3e).
The physical and chemical properties are closely related to the morphology and size of the nanomaterials. In our systems, the sizes of AuNPs in these supramolecular hybrids underwent significant changes after hydrolysis, naturally affecting their properties. As we know, one of the important applications of AuNPs is to catalyze reactions that are otherwise not feasible. Therefore, we examined the performance of these hybrids as catalysts for the borohydride reduction of 4-nitroaniline as a model reaction. The peak at 400 nm corresponding to the characteristic absorption of 4-nitroaniline remained unaltered for a long time in the absence of AuNPs, indicating that the reducing agent NaBH4 itself was unable to reduce 4-nitroaniline.16a On the contrary, the absorption band at 400 nm decreased gradually with the concomitant appearance of new peaks at 300 nm and 240 nm upon addition of the supramolecular hybrids (AuNPs@nanosheets or AuNPs@nanoparticles) into the reaction system, indicating that 1,4-diaminobenzene was produced (Fig. S32 and 31†), accompanied by a color change from yellow to transparent (Fig. 4). The kinetic reaction rate constants for the reduction with AuNPs@nanosheets and AuNPs@nanoparticles were estimated to be (1.85 ± 0.15) × 10−3 s−1 and (2.61 ± 0.28) × 10−3 s−1, respectively. Notably, the kinetic reaction rate constant of AuNPs@nanoparticles decreased to (1.78 ± 0.11) × 10−3 s−1 after treatment with AChE for 12 h (Fig. S34†), which was higher than that of AuNPs@nanosheets after the same treatment ((1.01 ± 0.09) × 10−3 s−1, Fig. S35†). The reasons was that the catalytic activity of the AuNPs is possibly through electron transfer from the BH4− anion to nitro compounds mediated by the large Fermi level shift of the nanoparticles.19a Compared with the AuNPs@nanosheets and AuNPs@nanoparticles, the sizes of AuNPs became larger due to the hydrolysis of PyCh by the AChE, resulting in the reduction of their specific surface area. For the AuNPs@nanoparticles, the hydrolyzate WP5⊃choline could act as clusters to protect AuNPs from aggregating to some extent, so the corresponding kinetic reaction rate was relatively larger.
Conclusions
In summary, a new pillar[5]arene/acetylcholine molecular recognition motif was established. Based on this molecular recognition motif, an enzyme-responsive supra-amphiphile was constructed by introducing an amphiphilic guest PyCh, which was sensitive to enzyme AChE. In contrast to the nanosheets self-assembled by PyCh alone through π–π stacking interactions between the pyrenyl groups, the host–guest complex WP5⊃PyCh self-assembled into nanoparticles induced by a curvature-dependent mechanism. PyCh was hydrolyzed into PyH and choline in the presence of AChE, resulting in the transformation of the nanosheets and nanoparticles into multi-layer nanoribbons. Intermolecular hydrogen bonds between the carboxylic acid groups on the surfaces of the nanoribbons played a significant role in the generation of these aggregates. Due to the existence of trimethylammonium groups on the surfaces of the nanosheets and negative carboxylate anions on the surfaces of the nanoparticles, supramolecular hybrid materials were prepared by introducing golden nanoparticles (AuNPs). Furthermore, these hybrids exhibited excellent catalytic abilities for the borohydride reduction of 4-nitroaniline as a model reaction. The specific surface areas of AuNPs in these supramolecular hybrids underwent significant changes after hydrolysis due to the growth of the AuNPs, resulting in the reduction of their catalytic abilities. These results exemplify the enormous potential of enzyme-responsive self-assembly for the construction of well-defined nanostructures that can be applied in many fields, including supramolecular polymers, nanoelectronics, and sensors.Experimental
Synthesis of 1
Methyl-6-bromohexanoate (5.16 g, 20.0 mmol) and K2CO3 (6.62 g, 48.0 mmol) were added to a solution of 1-pyrenol (2.18 g, 10.0 mmol) in CH3CN (100 mL). The mixture was heated in a three-necked flask under nitrogen atmosphere at reflux for 24 h. The cooled reaction mixture was filtered and washed with chloroform. The filtrate was evaporated under vacuum, and the residue was purified by flash column chromatography on silica gel (dichloromethane–petroleum ether = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, v/v) to afford 1 as a white solid (3.54 g, 83%), mp 112.5–113.8 °C. The proton NMR spectrum of 1 is shown in Fig. S1.† 1H NMR (400 MHz, chloroform-d, room temperature) δ (ppm): 8.45 (d, J = 8.0 Hz, 1H), 8.09 (t, J = 8.0 Hz, 3H), 8.01 (d, J = 8.0 Hz, 1H), 7.96–7.03 (m, 2H), 7.87 (d, J = 8.0 Hz, 1H), 7.51 (d, J = 8.0 Hz, 1H), 4.31 (t, J = 8.0 Hz, 2H), 3.68 (s, 3H), 2.40 (t, J = 8.0 Hz, 2H), 2.02–1.99 (m, 2H), 1.84–1.76 (m, 2H), 1.71–1.63 (m, 2H). The 13C NMR spectrum of 1 is shown in Fig. S2.† 13C NMR (125 MHz, chloroform-d, room temperature) δ (ppm): 174.11, 153.11, 131.76, 131.71, 127.26, 126.32, 126.08, 125.86, 125.49, 125.19, 124.99, 124.94, 124.22, 124.12, 121.24, 120.42, 109.12, 68.61, 51.55, 34.06, 29.21, 25.91, 24.80. LRESIMS is shown in Fig. S3:† m/z 346.9 [M + H]+ (100%). HRESIMS: m/z calcd for [M + H]+ C23H23O3, 347.1647, found 347.1655, error 2 ppm.
5, v/v) to afford 1 as a white solid (3.54 g, 83%), mp 112.5–113.8 °C. The proton NMR spectrum of 1 is shown in Fig. S1.† 1H NMR (400 MHz, chloroform-d, room temperature) δ (ppm): 8.45 (d, J = 8.0 Hz, 1H), 8.09 (t, J = 8.0 Hz, 3H), 8.01 (d, J = 8.0 Hz, 1H), 7.96–7.03 (m, 2H), 7.87 (d, J = 8.0 Hz, 1H), 7.51 (d, J = 8.0 Hz, 1H), 4.31 (t, J = 8.0 Hz, 2H), 3.68 (s, 3H), 2.40 (t, J = 8.0 Hz, 2H), 2.02–1.99 (m, 2H), 1.84–1.76 (m, 2H), 1.71–1.63 (m, 2H). The 13C NMR spectrum of 1 is shown in Fig. S2.† 13C NMR (125 MHz, chloroform-d, room temperature) δ (ppm): 174.11, 153.11, 131.76, 131.71, 127.26, 126.32, 126.08, 125.86, 125.49, 125.19, 124.99, 124.94, 124.22, 124.12, 121.24, 120.42, 109.12, 68.61, 51.55, 34.06, 29.21, 25.91, 24.80. LRESIMS is shown in Fig. S3:† m/z 346.9 [M + H]+ (100%). HRESIMS: m/z calcd for [M + H]+ C23H23O3, 347.1647, found 347.1655, error 2 ppm.
Synthesis of 2
A solution of 1 (3.46 g, 10.0 mmol) in CH3CH2OH (40 mL) was treated with 40% aqueous sodium hydroxide (80 mL) at reflux for 12 h. The mixture was concentrated under reduced pressure, diluted with water (10 mL), and acidified with HCl. The precipitated product 2 was collected by filtration, washed with water and dried under vacuum as a white solid (3.09 g, 93%), mp 126.8–128.3 °C. The proton NMR spectrum of 2 is shown in Fig. S4.† 1H NMR (400 MHz, DMSO-d6, room temperature) δ (ppm): 12.09 (s, 1H), 8.37 (d, J = 8.0 Hz, 1H), 8.24 (d, J = 8.0 Hz, 1H), 8.18 (t, J = 4.0 Hz, 2H), 8.13 (d, J = 8.0 Hz, 1H), 8.06 (t, J = 8.0 Hz, 2H), 8.01 (d, J = 8.0 Hz, 1H), 7.96 (d, J = 8.0 Hz, 1H), 7.75 (d, J = 8.0 Hz, 1H), 4.35 (t, J = 8.0 Hz, 2H), 2.29 (t, J = 8.0 Hz, 2H), 1.97–1.92 (m, 2H), 1.68–1.58 (m, 4H). The 13C NMR spectrum of 2 is shown in Fig. S5.† 13C NMR (100 MHz, DMSO-d6, room temperature) δ (ppm): 174.53, 152.67, 131.22, 131.08, 127.29, 126.37, 126.26, 126.00, 124.92, 124.62, 124.46, 124.30, 124.14, 124.09, 120.83, 119.33, 109.79, 68.37, 33.79, 28.63, 25.35, 24.39. LRESIMS is shown in Fig. S6:† m/z 330.9 [M − H]− (100%). HRESIMS: m/z calcd for [M − H]− C22H19O3, 331.1334, found 331.1341, error 2 ppm.Synthesis of 3
To a solution of 2 (1.66 g, 5.00 mmol) and N,N′-dimethylethanolamine (1.78 g, 20.0 mmol) in dry CH2Cl2 (100 mL), 4-dimethylaminopyridine (DMAP, catalytic amount) and 1-(3′-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC, 1.79 g, 10.0 mmol) were added under nitrogen atmosphere. The mixture was stirred overnight at room temperature. The solution was evaporated under vacuum and the residue was purified by flash column chromatography on silica gel (dichloromethane–petroleum ether = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) to afford 3 as a white solid (1.57 g, 78%), mp 104.1–106.4 °C. The proton NMR spectrum of 3 is shown in Fig. S7.† 1H NMR (400 MHz, chloroform-d, room temperature) δ (ppm): 8.45 (d, J = 8.0 Hz, 1H), 8.09 (t, J = 8.0 Hz, 2H), 8.03 (d, J = 8.0 Hz, 1H), 7.95–7.93 (m, 2H), 7.87 (d, J = 8.0 Hz, 1H), 7.51 (d, J = 8.0 Hz, 1H), 4.31 (t, J = 8.0 Hz, 2H), 4.31 (t, J = 8.0 Hz, 2H), 3.71 (t, J = 8.0 Hz, 2H), 2.55 (t, J = 8.0 Hz, 2H), 2.43 (t, J = 8.0 Hz, 2H), 2.26 (s, 6H), 1.84–1.64 (m, 6H). The 13C NMR spectrum of 3 is shown in Fig. S7.† 13C NMR (100 MHz, chloroform-d, room temperature) δ (ppm): 173.75, 153.11, 131.76, 131.71, 127.26, 126.32, 126.07, 125.85, 125.49, 125.18, 124.98, 124.21, 124.12, 121.25, 120.41, 109.12, 68.62, 57.81, 45.67, 34.21, 29.22, 25.89, 24.76, 18.43. LRESIMS is shown in Fig. S9:† m/z 404.0 [M + H]+ (100%). HRESIMS: m/z calcd for [M + H]+ C26H30NO3, 404.2226, found 404.2214, error −3 ppm.
1, v/v) to afford 3 as a white solid (1.57 g, 78%), mp 104.1–106.4 °C. The proton NMR spectrum of 3 is shown in Fig. S7.† 1H NMR (400 MHz, chloroform-d, room temperature) δ (ppm): 8.45 (d, J = 8.0 Hz, 1H), 8.09 (t, J = 8.0 Hz, 2H), 8.03 (d, J = 8.0 Hz, 1H), 7.95–7.93 (m, 2H), 7.87 (d, J = 8.0 Hz, 1H), 7.51 (d, J = 8.0 Hz, 1H), 4.31 (t, J = 8.0 Hz, 2H), 4.31 (t, J = 8.0 Hz, 2H), 3.71 (t, J = 8.0 Hz, 2H), 2.55 (t, J = 8.0 Hz, 2H), 2.43 (t, J = 8.0 Hz, 2H), 2.26 (s, 6H), 1.84–1.64 (m, 6H). The 13C NMR spectrum of 3 is shown in Fig. S7.† 13C NMR (100 MHz, chloroform-d, room temperature) δ (ppm): 173.75, 153.11, 131.76, 131.71, 127.26, 126.32, 126.07, 125.85, 125.49, 125.18, 124.98, 124.21, 124.12, 121.25, 120.41, 109.12, 68.62, 57.81, 45.67, 34.21, 29.22, 25.89, 24.76, 18.43. LRESIMS is shown in Fig. S9:† m/z 404.0 [M + H]+ (100%). HRESIMS: m/z calcd for [M + H]+ C26H30NO3, 404.2226, found 404.2214, error −3 ppm.
Synthesis of PyCh
A mixture of compound 3 (2.02 g, 5.00 mmol) and CH3I (5.68 g, 40.0 mmol) was heated in N,N′-dimethylformamide (50 mL) at 50 °C for 12 h. The solvent was evaporated, and the residue was washed with CH2Cl2 to give PyCh as a light yellow solid (1.66 g, 61%), mp 116.4–118.1 °C. The proton NMR spectrum of PyCh is shown in Fig. S10.† 1H NMR (400 MHz, DMSO-d6, room temperature) δ (ppm): 8.38 (d, J = 8.0 Hz, 1H), 8.25 (d, J = 8.0 Hz, 2H), 8.20 (t, J = 8.0 Hz, 2H), 8.14 (d, J = 8.0 Hz, 1H), 8.08 (d, J = 8.0 Hz, 1H), 8.03 (t, J = 8.0 Hz, 1H), 7.97 (d, J = 8.0 Hz, 1H), 7.76 (d, J = 8.0 Hz, 1H), 4.48–4.46 (m, 2H), 4.37 (t, J = 8.0 Hz, 2H), 3.67–3.65 (m, 2H), 2.45 (t, J = 8.0 Hz, 2H), 2.00–1.93 (m, 2H), 1.76–1.67 (m, 2H), 1.65–1.60 (m, 2H). The 13C NMR spectrum of PyCh is shown in Fig. S11.† 13C NMR (100 MHz, DMSO-d6, room temperature) δ (ppm): 172.32, 152.62, 131.22, 131.06, 127.28, 126.41, 126.27, 126.01, 124.93, 124.66, 124.49, 124.36, 124.11, 120.78, 119.32, 109.79, 68.30, 63.72, 57.69, 54.90, 52.90, 33.32, 28.55, 25.23, 24.01. LRESIMS is shown in Fig. S12:† m/z 418.0 [M − I]+ (100%). HRESIMS: m/z calcd for [M − I]+ C27H32NO3, 418.2382, found 418.2393, error 3 ppm.Critical aggregation concentration (CAC) determination
Some parameters such as the conductivity, osmotic pressure, fluorescence intensity and surface tension of the solution change sharply around the critical aggregation concentration. The dependence of the solution conductivity on the solution concentration is used to determine the critical aggregation concentration. Typically, the slope of the change in conductivity versus the concentration below CAC is steeper than the slope above the CAC. Therefore, the junction of the conductivity–concentration plot represents the CAC value. To measure the CAC value of PyCh (or WP5⊃PyCh), the conductivities of the solutions at different concentrations were determined. By plotting the conductivity versus the concentration, we estimated the CAC value of PyCh (or WP5⊃PyCh).Preparation of supramolecular hybrid materials
In a typical experiment, 0.10 mL of 1.0 × 10−4 M HAuCl4 and 6.0 mL of PyCh (or WP5⊃PyCh) aqueous solution were mixed in a 10 mL bottle. Then aqueous sodium borohydride (0.20 mL, 0.0125 g mL−1) was injected into the above solution under vigorous stirring. The solution became wine red, indicating that supramolecular hybrid materials AuNPs@nanosheets and AuNPs@nanoparticles were immediately obtained.Transmission electron microscopy (TEM) and dynamic light scattering (DLS) studies
The nanostructures of the self-assemblies were revealed using TEM. The concentrations of the solutions were higher than the corresponding critical aggregation concentrations of PyCh and WP5⊃PyCh. A solution of PyCh (or WP5⊃PyCh) was prepared first in water. TEM samples were prepared by drop-coating the solution on a carbon-coated copper grid. TEM experiments were performed on a HT-7700 instrument. The solution of WP5⊃PyCh was left to stand overnight and the insoluble precipitate was eliminated by using a microporous membrane before being used for DLS tests. The diameters of the assemblies were measured on a Nano-ZS ZEN3600 instrument.Catalytic reduction of 4-nitroaniline
The catalytic reduction of 4-nitroaniline was studied as follows. To a standard quartz cell with a 1 cm path length and about 4 mL volume, 3 mL of 0.20 mM 4-nitroaniline and 0.03 g of NaBH4 (large excess) were added. Then the addition of supramolecular hybrids (0.04 mL) to the mixture caused a decrease in the intensity of the absorption of 4-nitroaniline. The absorption spectra were recorded in a scanning range of 200–700 nm at room temperature. The control experiment was also carried out in the absence of supramolecular hybrids.Acknowledgements
This work was supported by the Fundamental Research Funds for the Central Universities.Notes and references
- (a) K. Wang, D.-S. Guo, X. Wang and Y. Liu, ACS Nano, 2011, 5, 2880–2894 CrossRef CAS PubMed; (b) C. Wang, Z. Wang and X. Zhang, Acc. Chem. Res., 2012, 45, 608–618 CrossRef CAS PubMed; (c) K. Wang, D.-S. Guo and Y. Liu, Chem.–Eur. J., 2012, 18, 8758–8764 CrossRef CAS PubMed.
- (a) F. Huang, F. R. Fronczek and H. W. Gibson, J. Am. Chem. Soc., 2003, 125, 9272–9273 CrossRef CAS PubMed; (b) F. Huang, M. Lam, E. J. Mahan, A. L. Rheingold and H. W. Gibson, Chem. Commun., 2005, 3268–3270 CAS; (c) H.-B. Yang, N. Das, F. Huang, A. M. Hawkridge, D. C. Muddiman and P. J. Stang, J. Am. Chem. Soc., 2006, 128, 10014–10015 CrossRef CAS PubMed; (d) H.-B. Yang, K. Ghosh, B. H. Northrop, Y.-R. Zheng, M. M. Lyndon, D. C. Muddiman and P. J. Stang, J. Am. Chem. Soc., 2007, 129, 14187–14189 CrossRef CAS PubMed; (e) L. Chen, Y.-K. Tian, Y. Ding, Y.-J. Tian and F. Wang, Macromolecules, 2012, 45, 8412–8419 CrossRef CAS; (f) L.-Y. Niu, Y.-S. Guan, Y.-Z. Chen, L.-Z. Wu, C.-H. Tung and Q.-Z. Yang, J. Am. Chem. Soc., 2012, 134, 18928–18931 CrossRef CAS PubMed; (g) H. Zhang, Q. Liu, J. Li and D.-H. Qu, Org. Lett., 2013, 15, 338–341 CrossRef CAS PubMed; (h) G.-Z. Zhao, L.-J. Chen, W. Wang, J. Zhang, G. Yang, D.-X. Wang, Y. Yu and H.-B. Yang, Chem.–Eur. J., 2013, 19, 10094–10100 CrossRef CAS PubMed; (i) X. Yan, D. Xu, J. Chen, M. Zhang, B. Hu, Y. Yu and F. Huang, Polym. Chem., 2013, 4, 3312–3322 RSC; (j) B. Xia, B. Zheng, C. Han, S. Dong, M. Zhang, B. Hu, Y. Yu and F. Huang, Polym. Chem., 2013, 4, 2019–2024 RSC.
- (a) H. Tian, B. Qin, R. Yao, X. Zhao and S. Yang, Adv. Mater., 2003, 15, 2104–2107 CrossRef CAS; (b) Y. Wang, N. Ma, Z. Wang and X. Zhang, Angew. Chem., Int. Ed., 2007, 46, 2823–2826 CrossRef CAS PubMed; (c) S. Silvi, A. Arduini, A. Pochini, A. Secchi, M. Tomasulo, F. M. Raymo, M. Baroncini and A. Credi, J. Am. Chem. Soc., 2007, 129, 13378–13379 CrossRef CAS PubMed; (d) J. Babin, M. Pelletier, M. Lepage, J.-F. Allard, D. Morris and Y. Zhao, Angew. Chem., Int. Ed., 2009, 48, 3329–3332 CrossRef CAS PubMed; (e) Q. Yan, J. Yuan, Z. Cai, Y. Xin, Y. Kang and Y. Yin, J. Am. Chem. Soc., 2010, 132, 9268–9270 CrossRef CAS PubMed; (f) X. Yan, S. Li, T. R. Cook, X. Ji, Y. Yao, J. B. Pollock, Y. Shi, G. Yu, J. Li, F. Huang and P. J. Stang, J. Am. Chem. Soc., 2013, 135, 14036–14039 CrossRef CAS PubMed; (g) X. Yan, B. Jiang, T. R. Cook, Y. Zhang, J. Li, Y. Yu, F. Huang, H.-B. Yang and P. J. Stang, J. Am. Chem. Soc., 2013, 135, 16813–16816 CrossRef CAS PubMed.
- (a) C. Park, H. Kim, S. Kim and C. Kim, J. Am. Chem. Soc., 2009, 131, 16614–16615 CrossRef CAS PubMed; (b) H. Koo, M. S. Huh, I.-C. Sun, S. H. Yuk, K. Choi, K. Kim and I. C. Kwon, Acc. Chem. Res., 2011, 44, 1018–1028 CrossRef CAS PubMed; (c) X. Huang, D. Appelhans, P. Formanek, F. Simon and B. Voit, ACS Nano, 2012, 6, 9718–9726 CrossRef CAS PubMed; (d) J. Zhuang, M. R. Gordon, J. Ventura, L. Li and S. Thayumanavan, Chem. Soc. Rev., 2013, 42, 7421–7435 RSC; (e) A. R. Rodriguez, J. R. Kramer and T. J. Deming, Biomacromolecules, 2013, 14, 3610–3614 CrossRef CAS PubMed.
- (a) V. Parikh, R. Kozak, V. Martinez and M. Sarter, Neuron, 2007, 56, 141–154 CrossRef CAS PubMed; (b) E. Eggermanna and D. Feldmeyer, Proc. Natl. Acad. Sci. U. S. A., 2009, 106, 11753–11758 CrossRef PubMed; (c) D.-S. Guo, V. D. Uzunova, X. Su, Y. Liu and W. M. Nau, Chem. Sci., 2011, 2, 1722–1734 RSC; (d) Y. Xing, C. Wang, P. Han, Z. Wang and X. Zhang, Langmuir, 2012, 28, 6032–6036 CrossRef CAS PubMed; (e) D.-S. Guo, T.-X. Zhang, Y.-X. Wang and Y. Liu, Chem. Commun., 2013, 49, 6779–6781 RSC; (f) Y.-L. Sun, Y. Zhou, Q.-L. Li and Y.-W. Yang, Chem. Commun., 2013, 49, 9033–9035 RSC; (g) D.-S. Guo, J. Yang and Y. Liu, Chem.–Eur. J., 2013, 19, 8755–8759 CrossRef CAS PubMed.
- (a) T. Ogoshi, Y. Nishida, T. Yamagishi and Y. Nakamoto, Macromolecules, 2010, 43, 7068–7072 CrossRef CAS; (b) Z. Zhang, B. Xia, C. Han, Y. Yu and F. Huang, Org. Lett., 2010, 12, 2385–2387 Search PubMed; (c) C. Li, L. Zhao, J. Li, X. Ding, S. Chen, Q. Zhang, Y. Yu and X. Jia, Chem. Commun., 2010, 46, 9016–9018 RSC; (d) W. Si, X.-B. Hu, X.-H. Liu, R. Fan, Z. Chen, L. Weng and J.-L. Hou, Tetrahedron Lett., 2011, 52, 2484–2487 CrossRef CAS PubMed; (e) Y. Yao, M. Xue, J. Chen, M. Zhang and F. Huang, J. Am. Chem. Soc., 2012, 134, 15712–15715 CrossRef CAS PubMed; (f) Z. Zhang, C. Han, G. Yu and F. Huang, Chem. Sci., 2012, 3, 3026–3031 RSC; (g) P. J. Cragg and K. Sharma, Chem. Soc. Rev., 2012, 41, 597–607 RSC; (h) H. Deng, X. Shu, X. Hu, J. Li, X. Jia and C. Li, Tetrahedron Lett., 2012, 53, 4609–4612 CrossRef CAS PubMed; (i) Y. Fang, L. Wu, J. Liao, L. Chen, Y. Yang, N. Liu, L. He, S. Zou, W. Feng and L. Yuan, RSC Adv., 2013, 3, 12376–12383 RSC; (j) H. Li, D.-X. Chen, Y.-L. Sun, Y. Zheng, L.-L. Tan, P. S. Weissv and Y.-W. Yang, J. Am. Chem. Soc., 2013, 135, 1570–1576 CrossRef CAS PubMed; (k) H. Zhang, K. T. Nguyen, X. Ma, H. Yan, J. Guo, L. Zhu and Y. Zhao, Org. Biomol. Chem., 2013, 11, 2070–2074 RSC; (l) T. Ogoshi and T. A. Yamagishi, Eur. J. Org. Chem., 2013, 2961–2975 CrossRef CAS; (m) J.-F. Xu, Y.-Z. Chen, L.-Z. Wu, C.-H. Tung and Q.-Z. Yang, Org. Lett., 2013, 15, 6148–6151 CrossRef CAS PubMed; (n) Y. Ma, M. Xue, Z. Zhang, X. Chi and F. Huang, Tetrahedron, 2013, 69, 4532–4535 CrossRef CAS PubMed; (o) C. Li, J. Ma, L. Zhao, Y. Zhang, Y. Yu, X. Shu, J. Li and X. Jia, Chem. Commun., 2013, 49, 1924–1926 RSC; (p) S. Dong, J. Yuan and F. Huang, Chem. Sci., 2014, 5, 247–252 RSC.
- (a) D. Cao, Y. Kou, J. Liang, Z. Chen, L. Wang and H. Meier, Angew. Chem., Int. Ed., 2009, 48, 9721–9723 CrossRef CAS PubMed; (b) C. Han, F. Ma, Z. Zhang, B. Xia, Y. Yu and F. Huang, Org. Lett., 2010, 12, 4360–4363 CrossRef CAS PubMed; (c) M. Xue, Y. Yang, X. Chi, Z. Zhang and F. Huang, Acc. Chem. Res., 2012, 45, 1294–1308 CrossRef CAS PubMed; (d) G. Yu, X. Zhou, Z. Zhang, C. Han, Z. Mao, C. Gao and F. Huang, J. Am. Chem. Soc., 2012, 134, 19489–19497 CrossRef CAS PubMed; (e) Y. Ma, X. Chi, X. Yan, J. Liu, Y. Yao, W. Chen, F. Huang and J.-L. Hou, Org. Lett., 2012, 14, 1532–1535 CrossRef CAS PubMed; (f) W. Chen, Y. Zhang, J. Li, X. Lou, Y. Yu, X. Jia and C. Li, Chem. Commun., 2013, 49, 7956–7958 RSC; (g) I. Nierengarten, S. Guerra, M. Holler, L. Karmazin-Brelot, J. Barberá, R. Deschenaux and J.-F. Nierengarten, Eur. J. Org. Chem., 2013, 3675–3684 CrossRef CAS; (h) C. Han, L. Gao, G. Yu, Z. Zhang, S. Dong and F. Huang, Eur. J. Org. Chem., 2013, 2529–2532 CrossRef CAS; (i) X. Chi, M. Xue, Y. Ma, X. Yan and F. Huang, Chem. Commun., 2013, 49, 8175–8177 RSC.
- (a) J. Terao, A. Tang, J. J. Michels, A. Krivokapic and H. L. Anderson, Chem. Commun., 2004, 56–57 RSC; (b) Q.-C. Wang, D.-H. Qu, J. Ren, K. Chen and H. Tian, Angew. Chem., Int. Ed., 2004, 43, 2661–2665 CrossRef CAS; (c) Y. J. Jeon, H. Kim, S. Jon, N. Selvapalam, D. H. Seo, I. Oh, C.-S. Park, S. Jung, R. D.-S. Koh and K. Kim, J. Am. Chem. Soc., 2004, 126, 15944–15945 CrossRef CAS PubMed; (d) W.-H. Huang, P. Y. Zavalij and L. Isaacs, Angew. Chem., Int. Ed., 2007, 46, 7425–7427 CrossRef CAS PubMed; (e) Y. Liu, A. Bruneau, J. He and Z. Abliz, Org. Lett., 2008, 10, 765–768 CrossRef CAS PubMed; (f) F. Wang, C. Han, C. He, Q. Zhou, J. Zhang, C. Wang, N. Li and F. Huang, J. Am. Chem. Soc., 2008, 130, 11254–11255 CrossRef CAS PubMed; (g) Z. Niu and H. W. Gibson, Chem. Rev., 2009, 109, 6024–6046 CrossRef CAS PubMed; (h) W. Jiang, A. Schäfer, P. C. Mohr and C. A. Schalley, J. Am. Chem. Soc., 2010, 132, 2309–2320 CrossRef CAS PubMed; (i) F. Wang, J. Zhang, X. Ding, S. Dong, M. Liu, B. Zheng, S. Li, K. Zhu, L. Wu, Y. Yu, H. W. Gibson and F. Huang, Angew. Chem., Int. Ed., 2010, 49, 1090–1094 CrossRef CAS PubMed; (j) Z. Niu, F. Huang and H. W. Gibson, J. Am. Chem. Soc., 2011, 133, 2836–2839 CrossRef CAS PubMed; (k) K. Zhu, V. N. Vukotic and S. J. Loeb, Angew. Chem., Int. Ed., 2012, 51, 2168–2172 CrossRef CAS PubMed; (l) D.-S. Guo and Y. Liu, Chem. Soc. Rev., 2012, 41, 5907–5921 RSC; (m) Z. Qi, P. M. Molina, W. Jiang, Q. Wang, K. Nowosinski, A. Schulz, M. Gradzielski and C. A. Schalley, Chem. Sci., 2012, 3, 2073–2082 RSC; (n) B. Zheng, F. Wang, S. Dong and F. Huang, Chem. Soc. Rev., 2012, 41, 1621–1636 RSC; (o) Y. Lan, X. J. Loh, J. Geng, Z. Walsh and O. A. Scherman, Chem. Commun., 2012, 48, 8757–8759 RSC; (p) B. Vinciguerra, L. Cao, J. R. Cannon, P. Y. Zavalij, C. Fenselau and L. Isaacs, J. Am. Chem. Soc., 2012, 134, 13133–13140 CrossRef CAS PubMed; (q) X. Yan, F. Wang, B. Zheng and F. Huang, Chem. Soc. Rev., 2012, 41, 6042–6065 RSC; (r) D.-S. Guo, K. Wang, Y.-X. Wang and Y. Liu, J. Am. Chem. Soc., 2012, 134, 10244–10250 CrossRef CAS PubMed; (s) Q. Zhang, D.-H. Qu, J. Wu, X. Ma, Q. Wang and H. Tian, Langmuir, 2013, 29, 5345–5350 CrossRef CAS PubMed.
- (a) J.-M. Liu, J.-H. Bu, Q.-Y. Zheng, C.-F. Chen and Z.-T. Huang, Tetrahedron Lett., 2006, 47, 1905–1908 CrossRef CAS PubMed; (b) S. Li, J. Chen, B. Zheng, S. Dong, Z. Ma, H. W. Gibson and F. Huang, J. Polym. Sci., Part A: Polym. Chem., 2010, 48, 4067–4073 CrossRef CAS; (c) J. Cao, Z.-P. Song, X.-Z. Zhu and C.-F. Chen, Tetrahedron Lett., 2010, 51, 3112–3115 CrossRef CAS PubMed; (d) S. Dong, Y. Luo, X. Yan, B. Zheng, X. Ding, Y. Yu, Z. Ma, Q. Zhao and F. Huang, Angew. Chem., Int. Ed., 2011, 50, 1905–1909 CrossRef CAS PubMed; (e) M. Zhang, D. Xu, X. Yan, J. Chen, S. Dong, B. Zheng and F. Huang, Angew. Chem., Int. Ed., 2012, 51, 7011–7015 CrossRef CAS PubMed; (f) S. Pan, D.-X. Wang, L. Zhao and M.-X. Wang, Tetrahedron, 2012, 68, 9464–9477 CrossRef CAS PubMed; (g) Y.-K. Tian, L. Chen, Y.-J. Tian, X.-Y. Wang and F. Wang, Polym. Chem., 2013, 4, 453–457 RSC; (h) X. Ji, Y. Yao, J. Li, X. Yan and F. Huang, J. Am. Chem. Soc., 2013, 135, 74–77 CrossRef CAS PubMed; (i) X. Ji, S. Dong, P. Wei, D. Xia and F. Huang, Adv. Mater., 2013, 25, 5725–5729 CrossRef CAS PubMed.
- (a) Z. Zhang, G. Yu, C. Han, J. Liu, X. Ding, Y. Yu and F. Huang, Org. Lett., 2011, 13, 4818–4821 CrossRef CAS PubMed; (b) L. Liu, L. Wang, C. Liu, Z. Fu, H. Meier and D. Cao, J. Org. Chem., 2012, 77, 9413–9417 CrossRef CAS PubMed.
- (a) N. L. Strutt, R. S. Forgan, J. M. Spruell, Y. Y. Botros and J. F. Stoddart, J. Am. Chem. Soc., 2011, 133, 5668–5671 CrossRef CAS PubMed; (b) G. Yu, Z. Zhang, C. Han, M. Xue, Q. Zhou and F. Huang, Chem. Commun., 2012, 48, 2958–2960 RSC.
- (a) Z. Zhang, Y. Luo, J. Chen, S. Dong, Y. Yu, Z. Ma and F. Huang, Angew. Chem., Int. Ed., 2011, 50, 1397–1401 CrossRef CAS PubMed; (b) Y. Guan, M. Ni, X. Hu, T. Xiao, S. Xiong, C. Lin and L. Wang, Chem. Commun., 2012, 48, 8532–8534 RSC; (c) X. Wang, K. Han, J. Li, X. Jia and C. Li, Polym. Chem., 2013, 4, 3998–4003 RSC; (d) X.-Y. Hu, X. Wu, S. Wang, D. Chen, W. Xia, C. Lin, Y. Pan and L. Wang, Polym. Chem., 2013, 4, 4292–4297 RSC.
- (a) W. Si, L. Chen, X.-B. Hu, G. Tang, Z. Chen, J.-L. Hou and Z.-T. Li, Angew. Chem., Int. Ed., 2011, 50, 12564–12568 CrossRef CAS PubMed; (b) X.-B. Hu, Z. Chen, G. Tang, J.-L. Hou and Z.-T. Li, J. Am. Chem. Soc., 2012, 134, 8384–8387 CrossRef CAS PubMed; (c) L. Chen, W. Si, L. Zhang, G. Tang, Z.-T. Li and J.-L. Hou, J. Am. Chem. Soc., 2013, 135, 2152–2155 CrossRef CAS PubMed.
- (a) Q. Duan, Y. Cao, Y. Li, X. Hu, T. Xiao, C. Lin, Y. Pan and L. Wang, J. Am. Chem. Soc., 2013, 135, 10542–10549 CrossRef CAS PubMed; (b) H. Zhang, X. Ma, K. T. Nguyen and Y. Zhao, ACS Nano, 2013, 7, 7853–7863 CrossRef CAS PubMed.
- G. Yu, Y. Ma, C. Han, Y. Yao, G. Tang, Z. Mao, C. Gao and F. Huang, J. Am. Chem. Soc., 2013, 135, 10310–10313 CrossRef CAS PubMed.
- (a) T. Ogoshi, R. Shiga and T. Yamagishi, J. Am. Chem. Soc., 2012, 134, 4577–4580 CrossRef CAS PubMed; (b) G. Yu, C. Han, Z. Zhang, J. Chen, X. Yan, B. Zheng, S. Liu and F. Huang, J. Am. Chem. Soc., 2012, 134, 8711–8717 CrossRef CAS PubMed; (c) G. Yu, M. Xue, Z. Zhang, J. Li, C. Han and F. Huang, J. Am. Chem. Soc., 2012, 134, 13248–13251 CrossRef CAS PubMed; (d) T. Ogoshi, K. Kida and T. Yamagishi, J. Am. Chem. Soc., 2012, 134, 20146–20150 CrossRef CAS PubMed; (e) W. Xia, X.-Y. Hu, Y. Chen, C. Lin and L. Wang, Chem. Commun., 2013, 49, 5085–5087 RSC.
- (a) B.-Y. Lu, G.-J. Sun, J.-B. Lin, X.-K. Jiang, X. Zhao and Z.-T. Li, Tetrahedron Lett., 2010, 51, 3830–3835 CrossRef CAS PubMed; (b) Z.-G. Tao, X. Zhao, X.-K. Jiang and Z.-T. Li, Tetrahedron Lett., 2012, 53, 1840–1842 CrossRef CAS PubMed; (c) X. Yan, D. Xu, X. Chi, J. Chen, S. Dong, X. Ding, Y. Yu and F. Huang, Adv. Mater., 2012, 24, 362–369 CrossRef CAS PubMed; (d) X. Yan, S. Li, J. B. Pollock, T. R. Cook, J. Chen, Y. Zhang, X. Ji, Y. Yu, F. Huang and P. J. Stang, Proc. Natl. Acad. Sci. U. S. A., 2013, 110, 15585–15590 CrossRef CAS PubMed; (e) F. Wang, M. Gillissen, P. J. M. Stals, A. R. A. Palmans and E. W. Meijer, Chem.–Eur. J., 2012, 18, 11761–11770 CrossRef CAS PubMed; (f) S. Dong, B. Zheng, D. Xu, X. Yan, M. Zhang and F. Huang, Adv. Mater., 2012, 24, 3191–3195 CrossRef CAS PubMed; (g) X. Ji, J. Li, J. Chen, X. Chi, K. Zhu, X. Yan, M. Zhang and F. Huang, Macromolecules, 2012, 45, 6457–6463 CrossRef CAS; (h) T.-Y. Zhou, F. Lin, Z.-T. Li and X. Zhao, Macromolecules, 2013, 46, 7745–7752 CrossRef CAS.
- (a) K. Liu, Y. Yao, Y. Liu, C. Wang, Z. Li and X. Zhang, Langmuir, 2012, 28, 10697–10702 CrossRef PubMed; (b) K. Liu, Y. Yao, C. Wang, Y. Liu, Z. Li and X. Zhang, Chem.–Eur. J., 2012, 18, 8622–8628 CrossRef CAS PubMed.
- (a) Y. Yao, M. Xue, X. Chi, Y. Ma, J. He, Z. Ablizb and F. Huang, Chem. Commun., 2012, 48, 6505–6507 RSC; (b) Y. Yao, M. Xue, Z. Zhang, M. Zhang, Y. Wang and F. Huang, Chem. Sci., 2013, 4, 3667–3672 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Synthetic procedures, characterizations, and other materials. See DOI: 10.1039/c4ra01820f |
| This journal is © The Royal Society of Chemistry 2014 |




