Effective hydrodechlorination of 4-chlorophenol catalysed by magnetic palladium/reduced graphene oxide under mild conditions
Abstract
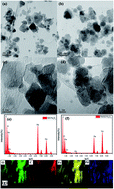
Magnetic palladium/reduced graphene oxide nanocomposites (Pd/rGO-Fe3O4) were prepared by depositing Pd nanoparticles on an rGO-Fe3O4 magnetic support. The as-prepared nanocomposites were investigated as catalysts for the hydrodechlorination of 4-chlorophenol in the aqueous phase. The complete conversion of 4-chlorophenol (with a concentration as high as 2.5 g L−1) to phenol was obtained in a reaction time of 40 min at room temperature and balloon hydrogen pressure without any additives. The excellent catalytic activity of the Pd/rGO-Fe3O4 can be attributed to the small particle size of Pd, and an electron-deficient state of Pd in the catalyst as a result of the strong interaction between the active sites and the oxygen of the oxygen-containing groups on the rGO-Fe3O4 support.

 Please wait while we load your content...
Please wait while we load your content...