Study of fuchsine adsorption on magnetic chitosan/graphene oxide†
Leilei Li,
Lulu Fan,
Chuannan Luo*,
Huimin Duan and
Xiaojiao Wang
Key Laboratory of Chemical Sensing & Analysis in Universities of Shandong (University of Jinan), School of Chemistry and Chemical Engineering, University of Jinan, Jinan 250022, China. E-mail: haoyunlileilei@163.com; Tel: +86 53189736065
First published on 7th May 2014
Abstract
Magnetic chitosan/graphene oxide (MCGO) was used as a potential adsorbent for fuchsine removal from aqueous systems. The magnetic composite bioadsorbent was characterized by SEM, TEM, FTIR and XRD measurements. The large saturation magnetization (48.93 emu g−1) of the synthesized nanoparticles allowed fast separation of MCGO from the liquid suspension. Adsorption data were well studied for adsorption isotherms and kinetics models. The adsorption of fuchsine followed second-order kinetics and was best fitted to the Langmuir adsorption isotherms. MCGO displayed excellent stability over 5 cycles of use and regeneration without incurring significant structural damage or a decrease in fuchsine adsorption properties. These results suggested that the new developed material is a potential adsorbent for the effective removal of dyes from aquatic solutions.
1. Introduction
Dye pollution has led to severe ecological and environmental problems because most of the used dyes are toxic, and some are considered carcinogenic for human health.1 Therefore, the removal of dyes from aqueous solutions is of important interest in view of environmental risks. Some treatment technologies have been developed for dye removal from water, such as reverse osmosis, adsorption, precipitation and ion exchange.2–10 However, some treatment methods are ineffective for dye removal because of the complex aromatic structures and xenobiotic properties of dyes.11 Among these investigated methods, adsorption is widely applicable for the eradication of dyes due to its simplicity, economic viability and the availability of different adsorbents.12 If a high-efficiency adsorbent is chosen for a target species, effective removal can be performed. Thus, particular attention has been paid in recent years to the development of microporous adsorbents with a high specific surface area and surface functional groups.13 These properties proved to be advantageous by increasing the adsorption capacity and facilitating the recycling of the adsorbent for further adsorption of dyes from water.Graphene is a fascinating new member of carbon materials with a honeycomb, one-atom-thick structure and high specific surface area.14,15 Recent research has indicated that graphene proved to be a promising material to adsorb dyes and support for catalysts due to its extraordinary mechanical strength, relatively large specific area, and intrinsically hydrophobic surface; however, the poor dispersibility of graphene in solution and the low adsorbent yield per unit mass have hindered its adoption in applications, particularly in the context of environmental remediation.14,16–18 To overcome these limitations, an alternative material, graphene oxide (GO), has been tested as a scalable precursor for preparing graphene-based bulk materials.19–25
Recently, magnetic chitosan nanoparticles have attracted tremendous attention due to their many excellent properties arising from either the components themselves or functionalization and synergy,15,16 such as (a) magnetic chitosan remarkably enhances the stability of chitosan in solution; (b) magnetic biosorbents are easy to separate and recover; (c) high surface area and surface functional groups improve the adsorption capacity of chitosan. Magnetic chitosan can be as an effective biosorbent in removing dyes from effluents. Furthermore, the fabrication of magnetic chitosan/graphene oxide nanocomposite (MCGO), especially those with great potential, can be used in environmental remediation with fast treatment.
The objectives of this study were (a) to prepare MCGO composites and to characterize them by SEM, TEM, FTIR, VSM and XRD; (b) to study the effects of treatment time and pH on fuchsine removal; (c) to simulate fuchsine adsorption isotherms and to estimate adsorption capacities; and (d) to evaluate the possibility of regeneration and reusability of MCGO as an adsorbent. MCGO are found to possess a unique capability to remove fuchsine rapidly and efficiently from wastewater. MCGO could be repeatedly used by simple treatment without obvious structure and performance degradation. The application of MCGO for the removal of fuchsine with the help of an external magnetic field is shown in Scheme 1.
 | ||
| Scheme 1 Synthesis of MCGOs and their application for the removal of fuchsine with the help of an external magnetic field. | ||
2. Materials and methods
2.1. Materials
Chitosan was purchased from Qingdao Baicheng Biochemical Corp. (China). Reagents 1-ethyl-3-(3-dimethylaminoprophy) carbodiimide hydrochloride (EDC), N-hydroxyl succinimide (NHS), sodium hydroxide, glutaraldehyde and acetic acid were obtained from Aldrich. FeCl2·4H2O and FeCl3·6H2O were purchased from Damao Chemical Agent Company (Tijin, China). All other reagents used in this study were of analytical grade, and distilled or double distilled water was used in the preparation of all solutions.2.2. Preparation of magnetic chitosan
A solution of FeCl2·4H2O, FeCl3·6H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) in 25 mL of double distilled water was added dropwise to an ammonia solution, which was purged with nitrogen and stirred in a water bath at 85 °C for 3.5 h. Magnetic particles used in the chitosan coating were obtained by magnetic separation. Chitosan (0.3 g) was dissolved in the acetic solution (30 mL 3%) to give a final concentration of 1.5% (w/v). Magnetic particles (0.1 g) were added in the chitosan solution in a four-neck round bottom flask. Pure glutaraldehyde (2.0 mL) was added to the reaction flask and was stirred at 60 °C for 2 h. The precipitate was washed with petroleum ether, ethanol and distilled water in turn until pH was about 7. Then, the precipitate was dried in a vacuum oven at 50 °C. The obtained product was magnetic chitosan.26
2) in 25 mL of double distilled water was added dropwise to an ammonia solution, which was purged with nitrogen and stirred in a water bath at 85 °C for 3.5 h. Magnetic particles used in the chitosan coating were obtained by magnetic separation. Chitosan (0.3 g) was dissolved in the acetic solution (30 mL 3%) to give a final concentration of 1.5% (w/v). Magnetic particles (0.1 g) were added in the chitosan solution in a four-neck round bottom flask. Pure glutaraldehyde (2.0 mL) was added to the reaction flask and was stirred at 60 °C for 2 h. The precipitate was washed with petroleum ether, ethanol and distilled water in turn until pH was about 7. Then, the precipitate was dried in a vacuum oven at 50 °C. The obtained product was magnetic chitosan.26
2.3. Synthesis of graphene oxide
Graphene oxide was prepared from purified natural graphite by the modified Hummers method.27 Briefly, natural graphite and potassium permanganate were stirred with the mixed acid (H2SO4–HNO3 = 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) for 12 h at 60 °C. Then, hydrogen peroxide was added to the mixed liquor and stirred for 1 hour, resulting in a bright yellow colored end product.28 This material was washed with 2 M HCl until bisulfate ions were removed and then with copious amount of water till the solution became neutral. Subsequently, GO obtained by centrifugation was dried in a vacuum desiccator.
1) for 12 h at 60 °C. Then, hydrogen peroxide was added to the mixed liquor and stirred for 1 hour, resulting in a bright yellow colored end product.28 This material was washed with 2 M HCl until bisulfate ions were removed and then with copious amount of water till the solution became neutral. Subsequently, GO obtained by centrifugation was dried in a vacuum desiccator.
2.4. Preparation of MCGO
A GO dispersion was prepared by sonicating GO for 3 h in ultrapure water. A solution of EDC (0.05 M) and NHS (0.05 M) was added to the GO dispersion with continuous stirring for 2 h in order to activate the carboxyl groups of GO.29 The pH of the resulting solution was maintained at 7.0 using dilute sodium hydroxide. Magnetic chitosan (0.1 g) and the activated GO solution were added in a flask and dispersed in distilled water by ultrasonic dispersion for 10 min. After ultrasonic dispersion, the mixed solution was stirred at 60 °C for 2 h. The precipitate was washed with 2% (w/v) NaOH and distilled water in turn until pH was about 7. Then, the obtained product was collected by the aid of an adscititious magnet and dried in a vacuum oven at 50 °C. The obtained product was MCGO. The preparation of MCGO is shown in Scheme 1.2.5. Adsorption experiments
Batch adsorption experiments were carried out by using MCGO as the adsorbent. All batch adsorption experiments were performed on a SHA-C shaker (Changzhou, China) with a shaker speed of 160 rpm until the system reached equilibrium. Typically, a 25 mL solution of known fuchsine concentration and 0.02 g of MCGO were added into 100 mL glass flasks and then shook under 30 ± 0.2 °C. At the completion of preset time intervals, the dispersion was drawn and separated immediately by the aid of a magnet to collect the bioadsorbent. The residual fuchsine concentration in the supernatant was measured using a spectrophotometer (722-type, Shanghai Analytical Instruments General Factory, China). The adsorption amount and adsorption rate are calculated based on the difference in the fuchsine concentration in the aqueous solution before and after adsorption, according to the following equation,| Q = (C0 − Ce)V/W, E = (C0 − Ce)/C0 × 100% |
2.6. Characterization of the samples
The microscopic observation of samples was carried out using a scanning electron microscope (S-2500, Japan Hitachi). FTIR spectra of samples were recorded with a KBr pellet in the range of 400–4000 cm−1. FTIR spectra were measured on a Nicolet Magna 550 spectrometer. Wide angle X-ray diffraction (WAXRD) patterns were recorded by a D8 ADVANCE X-ray diffraction spectrometer (Bruker, German) with a Cu Kα target at a scan rate of 0.02° 2θ s−1 from 5° to 70°. The Brunauer–Emmett–Teller (BET) surface area and the pore size distribution of the composite were measured using N2 adsorption and desorption (QUADRASORBSI, Quantachrome, USA) at 77 K over a relative pressure ranging from 0.0955 to 0.993.2.7. Replication of batch experiment
Each batch adsorption experiment above was conducted in triplicate to obtain reproducible results with an error of <5%. In the case of deviations larger than 5%, more tests were carried out. The experimental data could be reproduced with an accuracy greater than 95%. All the data of batch adsorption experiments listed in Section 3 are the average values of three tests.3. Results and discussion
3.1 Structural characterizations of MCGO materials
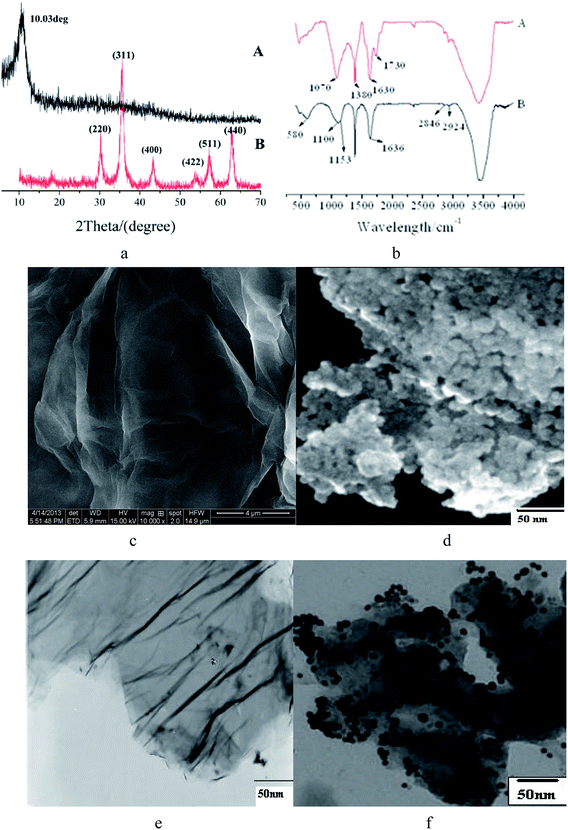
The results of XRD, FTIR, SEM, and TEM characterization of the prepared MCGO are shown in Fig. 1. XRD patterns of pure GO (A) and MCGO (B) are shown in Fig. 1a. As shown in Fig. 1a(A), the broad and relatively weak diffraction peak at 2θ = 10.03° (d = 0.87 nm), which corresponds to the typical diffraction peak of graphene oxide nanosheets, is attributed to the (002) plane. The c-axis spacing increases from 0.34 to 0.87 nm after graphite is modified to graphene oxide nanosheets, which is due to the creation of the abundant oxygen-containing functional groups on the surfaces of graphene oxide nanosheets.30 The XRD pattern of MCGO is shown in Fig. 1a(B), indicating the existence of iron oxide particles (Fe3O4). XRD analysis results of pure Fe3O4 and MCGO were mostly coincident. Six characteristic peaks for Fe3O4 (2θ = 30.1, 35.5, 43.3, 53.4, 57.2 and 62.5), marked by their indices ((220), (311), (400), (422), (511) and (440)), were observed in samples. The magnetization property of MCGO was investigated at room temperature by measuring the magnetization curve. The saturation magnetization (Ms) of MCGO is 48.93 emu g−1, indicating that MCGO is highly magnetic.The FTIR pattern of GO, as shown in Fig. 1b(A), reveals the presence of the oxygen-containing functional groups. The peaks at 1070, 1380 and 1630 cm−1 correspond to C–O–C stretching vibrations, C–OH stretching and C–C stretching mode of the sp2 carbon skeletal network, respectively, while peaks located at 1730 and 3440 cm−1 correspond to C–O stretching vibrations of the –COOH groups and O–H stretching vibration, respectively.29 As shown in Fig. 1b(B), there are two characteristic absorbance bands centered at 1636 and 1597 cm−1, which correspond to the C–O stretching vibration of –NHCO– (amide I) and N–H bending of –NH2, respectively.31 However, in the case of GO-grafted derivatives, it can be distinctly observed that the –NH2 absorbance band has shifted to a lower value, and the intensity of the acetylated amino group –NHCO– (amide I) has increased, which proves that –NH2 groups on the chitosan chains have reacted with the –COOH groups of GO and therefore have been converted to –NHCO– graft points. In addition, 580 cm−1 is the characteristic peak of Fe3O4. These indicate that magnetic chitosan was successfully grafted on GO.
Fig. 1c shows the typical SEM images of GO obtained by the modified Hummers method. Fig. 1e shows a typical TEM image of the GO; it presents a sheet-like structure with a large thickness, smooth surface and wrinkled edge. After combination with magnetic chitosan to form the MCGO composite (Fig. 1d), MCGO had a much rougher surface. Fig. 1f reveals that many small magnetic chitosan chains had been assembled on the surface of GO layers with a high density.
The BET surface area and pore volume of MCGO estimated from Barrett–Joyner–Halenda (BJH) analysis of the isotherms were determined to be 347.5 m2 g−1 and 0.3899 cm3 g−1, respectively. Also, the average of the pore size distribution was 2.987 nm. The value of the pore diameter indicates that MCGO was a mesoporous material; the diffusion parameter of MCGO is about 2.36 by calculation.
3.2. Effect of pH value on adsorption
The initial pH of the aqueous solution was an important parameter for determining the adsorption capacity on MCGO. The experiments were carried out in the pH range 3.0–7.5, and the results are illustrated in Fig. 2. Fuchsine uptake was increased as pH increased from 3.0 to 5.5. Fuchsine is a weak basic compound containing amido groups. At lower pH values, because of the protonation of the amido groups, fuchsine can be ionized, and consequently, its solubility in water can increase, which can result in its decreased uptake on MCGO. Above pH 5.5, MCGO displayed a decrease in the uptake value as pH increased. Therefore, the optimum pH range for fuchsine adsorption on MCGO was 5.5–6.5. This may be due to the more functional groups formed on the surface of MCGO, which increase their surface complexation capability.32,33 Chitosan becomes strongly anionic after grafting with GO.34 By increasing the pH of solution, the deprotonation of the (MCGO) derivative is realized, and strong attractive forces between the positive charged dye and negatively charged MCGO result in high uptake as follows:35,36| RCOO− + D–NH3+ → RCOO−D–NH3+ |
| –CH2O− + D–NH3+ → –CH2O−D–NH3+ |
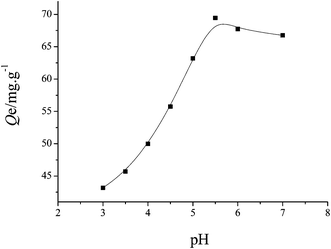 | ||
| Fig. 2 Effect of solution pH on fuchsine removal efficiency of MCGO. The concentration of MCGO was 1.0 g L−1. Initial fuchsine concentrations were 100 mg L−1, temperature: 303 K. | ||
Thus, a pH of about 5.5 was selected as the optimum pH value for the fuchsine solution for the following adsorption experiments.
3.3. Adsorption kinetics
The kinetics of adsorption describing the fuchsine uptake rate is one of the important characteristics that control the residence time of adsorbate uptake at the solid–liquid interface. The effect of the contact time for MCGO on the adsorption capacity for fuchsine is described in Fig. 3a. Obviously, MCGO showed a good performance in adsorption during the first 40 min. Quantifying the changes in adsorption with time requires appropriate kinetic models (pseudo-first order and pseudo-second order), which were investigated and compared. The pseudo-first order equation of Lagergren is expressed as follows:| log(qe − qt) = log(qe) − (k1/2.303)t |
The adsorption data of fuchsine at different time intervals fit a pseudo-second-order kinetic model. The pseudo-second-order model is expressed by
| t/Qt = 1/(K2Qe2) + t/Qe |
3.4. Evaluation of adsorption isotherm models
There are two adsorption isotherm models to study the fuchsine adsorption equilibrium. The Langmuir adsorption isotherm has been used to fit the experimental adsorption data for fuchsine on MCGO. The Langmuir adsorption isotherm is based on the assumption that adsorption takes place on a homogeneous surface. The equation can be expressed as| Ce/Qe = 1/(KLQ0) + Ce/Q0 |
The linear form of the Freundlich model could be expressed as follows:
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) qe = ln qe = ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KF + (1/n)ln KF + (1/n)ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ce ce |
 | ||
| Fig. 4 Linear dependence of Ce/Qe on Ce. The concentration of MCGO was 1.0 g L−1; pH, 5.5; temperature, 303 K; contact time, 60 min. | ||
By calculating, the results are as follows:
| Ce/Qe = 0.014Ce + 0.15, (R2 = 0.99), Q0 = 71.42 mg g−1, KL = 0.09 L mg−1 |
The values of Q0 obtained from Langmuir curves are mainly consistent with that experimentally obtained (75.31 mg g−1), indicating that the adsorption process is mainly monolayer. The strong adsorption by MCGO benefits from the large surface area and the abundant groups (carboxyl, hydroxyl, and epoxy functional groups). The chelation adsorption mechanism for fuchsine may give controlled monolayer adsorption.
Furthermore, the essential characteristics of the Langmuir isotherm can be described by a separation factor, which is defined by the following equation:
| RL = 1/(1 + KLCe) |
The value of RL indicates the shape of the Langmuir isotherm and the nature of the adsorption process. It is considered to be a favorable process when the value is within the range 0–1. In the study, the value of RL calculated for the initial concentrations of fuchsine was 0.099. Since the result is within the range of 0–1, the adsorption of fuchsine onto the adsorbent appears to be a favorable process. In addition, the low RL values (<0.1) implied that the interaction of fuchsine with MCGO might be relatively strong.
3.5. Effect of recycling adsorbents for fuchsine adsorption
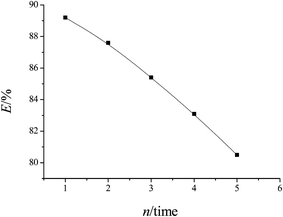
For practical application, recycling and regeneration of the adsorbent is indispensable. Because of magnetic properties, the collection of fuchsine-adsorbed MCGO was easy and fast. The fuchsine-adsorbed MCGO was treated with ethanol solution.The effect of five consecutive adsorption–desorption cycles was studied, and the results are shown in Fig. 5. It is shown in Fig. 5 that the adsorption capacity of fuchsine on the adsorbents decreased slowly with increasing cycle number. It is found that the removal efficiency was 89% at the first cycle and then slightly deceased to 80% at the fifth cycle. Our recyclability studies suggest that the nanoadsorbents can be repeatedly used as efficient adsorbents in wastewater treatment.
The slight removal efficiency decrease over five consecutive cycles of adsorption can be explained well: (1) fuchsine adsorbed on MCGO cannot be completely desorbed. With the increase in the adsorbed fuchsine remaining on the surface of MCGO, the adsorption capacity would decrease; (2) it is known that the active sites of MCGO are the surface hydroxyl groups. The abundant groups decreased, causing less fuchsine to be adsorbed by the used MCGO.
4. Conclusions
A recyclable and efficient graphene-based adsorbent (MCGO) is prepared via a simple chemical synthesis method. The structures and physical properties of the materials, including the adsorption properties, were investigated. MCGOs are superparamagnetic at room temperature and can be separated by an external magnetic field. MCGOs are highly stable even when immersed in 1 M HCl aqueous acid. MCGO exhibits a relatively high removal rate and higher adsorption capacity for solutions at pH 5. The pseudo-second-order kinetic model best describes the adsorption behavior of fuchsine on MCGO. The materials were remarkably recyclable over more than five adsorption–desorption cycles. These results suggest that this preparation method may be readily extended to the commercial production of graphene-based adsorbents for the sequestration of a variety of pollutants.Acknowledgements
This work was supported by the National Natural Science Foundation of China (NSFC, no. 21345005 and 21205048), the Shandong Provincial Natural Science Foundation of China (no. ZR2012BM020) and the Scientific and technological development Plan Item of Jinan City in China (no. 201202088).References
- R. D. Ambashta and M. Sillanpϋ, J. Hazard. Mater., 2010, 180, 38–49 CrossRef CAS PubMed.
- T. Zhang, L. L. Ding, H. Q. Ren and X. Xiong, Water Res., 2009, 43, 5209 CrossRef CAS PubMed.
- J. R. Kumar, H. I. Lee, J. Y. Lee, J. S. Kim and J. S. Sohn, Sep. Purif. Technol., 2008, 63, 184 CrossRef CAS PubMed.
- J. Mansouri, S. Harrisson and V. Chen, J. Mater. Chem., 2010, 20, 4567 RSC.
- Y. Tian, L. Chen and T. L. Jiang, Ind. Eng. Chem. Res., 2011, 50, 1127 CrossRef CAS.
- L. He, D. Li, G. Y. Zhang, P. A. Webley, D. Y. Zhao and H. T. Wang, Ind. Eng. Chem. Res., 2010, 49, 4175 CrossRef CAS.
- O. N. Kononova, T. A. Leyman, A. M. Melnikov, D. M. Kashirin and M. M. Tselukovskaya, Hydrometallurgy, 2010, 100, 161 CrossRef CAS PubMed.
- X. G. Li, R. Liu and M. R. Huang, Chem. Mater., 2005, 17, 5411 CrossRef CAS.
- S. Wang and Z. H. Zhu, J. Hazard. Mater. B, 2006, 136, 946 CrossRef CAS PubMed.
- H. Y. Zhu and R. L. Jiang, Bioresour. Technol., 2010, 101, 5063–5069 CrossRef CAS PubMed.
- K. M. Parida, S. Sahu, K. H. Reddy, P. C. Sahoo and A. Kinetic, Ind. Eng. Chem. Res., 2011, 50, 843–848 CrossRef CAS.
- V. K. Gupta and A. Rastogi, J. Hazard. Mater., 2009, 163, 396–402 CrossRef CAS PubMed.
- A. Li, H. X. Sun, D. Z. Tan, W. J. Fan, S. H. Wen, X. J. Qing, G. X. Li, S. Y. Li and W. Q. Deng, Energy Environ. Sci., 2011, 4, 2062 CAS.
- S. J. Yang, J. H. Kang, H. Jung, T. Kim and C. R. Park, J. Mater. Chem. A, 2013, 1, 9427–9432 CAS.
- L. Fan, C. N. Luo, M. Sun and X. J. Li, Colloids Surf., B, 2012, 95, 42–49 CrossRef CAS PubMed.
- Y. J. Guo, S. J. Guo, Y. M. Zhai, S. J. Dong and E. K. Wang, ACS Nano, 2010, 4, 4001–4010 CrossRef CAS PubMed.
- G. Srinivas, Y. Zhu, R. Piner, N. Skipper, M. Ellerby and R. Ruoff, Carbon, 2010, 48, 630 CrossRef CAS PubMed.
- H. K. Chae, D. Y. Siberio-Perez, J. Kim, Y. Go, M. Eddaoudi, A. J. Matzger, M. O'Keeffe and O. M. Yaghi, Nature, 2004, 427, 523 CrossRef CAS PubMed.
- F. Kim, L. J. Cote and J. Huang, Adv. Mater., 2010, 22, 1954 CrossRef CAS PubMed.
- H. B. Zhang, J. W. Wang, Q. Yan, W. G. Zheng, C. Chen and Z. Z. Yu, J. Mater. Chem., 2011, 21, 5392 RSC.
- D. Kim, S. J. Yang, Y. S. Kim, H. Jung and C. R. Park, Carbon, 2012, 50, 3229 CrossRef CAS PubMed.
- J. E. Kim, T. H. Han, S. H. Lee, J. Y. Kim, C. W. Ahn, J. M. Yun and S. O. Kim, Angew. Chem., Int. Ed., 2011, 50, 3043 CrossRef CAS PubMed.
- U. Maitra, H. S. S. R. Matte, P. Kumar and C. N. R. Rao, Chimia, 2012, 66, 941 CrossRef CAS PubMed.
- P. Kumar, K. S. Subrahmanyam and C. N. R. Rao, Int. J. Nanosci., 2011, 10, 559 CrossRef CAS.
- P. Kumar, B. Das, B. Chitara, K. S. Subrahmanyam, K. Gopalakrishnan, S. B. Krupanidhi and C. N. R. Rao, Macromol. Chem. Phys., 2012, 213, 1146 CrossRef CAS.
- L. Fan, et al., J. Hazard. Mater., 2012, 215–216, 272–279 CrossRef CAS PubMed.
- P. Ramesh, S. Bhagyalakshmi and S. Sampath, J. Colloid Interface Sci., 2004, 274, 95 CrossRef CAS PubMed.
- G. K. Ramesha, A. Vijaya Kumara, H. B. Muralidhara and S. Sampath, J. Colloid Interface Sci., 2011, 361, 270–277 CrossRef CAS PubMed.
- D. Depan, B. Girase, J. S. Shah and R. D. K. Misra, Acta Biomater., 2011, 7, 3432–3445 CrossRef CAS PubMed.
- A. Lerf, H. Y. He, M. Forster and J. Klinowski, J. Phys. Chem. B, 1998, 102, 4477–4482 CrossRef CAS.
- M. Sigomoto, M. Morimoto, H. Sashiwa, H. Saimoto and Y. Shigemasa, Carbohydr. Polym., 1998, 36, 49–59 CrossRef.
- Y. H. Li, Q. J. Du, X. D. Wang, P. D. Zhang, C. Wang, Z. H. Wang and Y. Z. Xia, J. Hazard. Mater., 2010, 177, 876–880 CrossRef CAS PubMed.
- G. Z. Kyzas and N. K. Lazaridis, J. Colloid Interface Sci., 2009, 331, 32–39 CrossRef CAS PubMed.
- S. Benamer, M. Mahlous, D. Tahtat, A. Nacer-Khodja, M. Arabi, H. Lounici and N. Mameri, Radiat. Phys. Chem., 2011, 12, 1391–1397 CrossRef PubMed.
- G. Crini, F. Gimbert, C. Robert, B. Martel, O. Adam, N. Morin-Crini, F. De Giorgi and P.-M. Badot, J. Hazard. Mater., 2008, 153, 96–106 CrossRef CAS PubMed.
- N. K. Lazaridis, G. Z. Kyzas, A. A. Vassiliou and D. N. Bikiaris, Langmuir, 2007, 23, 7634–7643 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra01784f |
| This journal is © The Royal Society of Chemistry 2014 |