Thermal conductivities, mechanical and thermal properties of graphite nanoplatelets/polyphenylene sulfide composites
Junwei Gu*ab,
Junjie Duab,
Jing Dangab,
Wangchang Gengab,
Sihai Huab and
Qiuyu Zhang*ab
aKey Laboratory of Space Applied Physics and Chemistry, Ministry of Education, Department of Chemistry, School of Science, Northwestern Polytechnical University, Xi'an, 710072, P. R. China. E-mail: nwpugjw@163.com; qyzhang1803@gmail.com; Fax: +86-29-88431675; Tel: +86-29-88431675
bSchool of Science, Northwestern Polytechnical University, Youyi Road 127#, Xi'an, 710072, P. R. China
First published on 7th May 2014
Abstract
Functionalized pristine graphite nanoplatelets (fGNPs) by methanesulfonic acid/isopropyltrioleictitanate (MSA/NDZ-105) are used to fabricate fGNPs/polyphenylene sulfide (fGNPs/PPS) composites by mechanical ball milling followed by a compression molding method. The thermal conductive coefficient of the fGNPs/PPS composite with 40 wt% fGNPs is greatly improved to 4.414 W m−1 K−1, 19 times higher than that of the original PPS. For a given GNP loading, the surface functionalization of GNPs by MSA/NDZ-105 results in the fGNPs/PPS composites improving thermal conductivities by minimizing the interfacial thermal resistance. The thermal stabilities of the fGNPs/PPS composites are increased with the increasing addition of fGNPs.
Introduction
The continuing miniaturization of microelectronic devices is setting higher and stricter requirements for performance, reliability, and processing techniques for advanced packaging materials.1–8 In this context, high thermal conductivity polymers are promising candidates to address heat dissipation problems in microelectronic packaging and printed circuit boards.9,10 However, the thermally conductive coefficient of most polymers is relatively low.Recent studies have shown that by incorporation thermally conductive fillers, such as carbon nanotube,11–14 boron nitride nanotube,15 silicon nitride,16,17 silicon carbide,18,19 graphite20–22 and carbon black23,24 into polymer matrix can increase the thermal conductivities of the polymeric composites. In our previous research,25–28 several different thermal conductivity composites have also been fabricated successfully by adding various single or hybrid thermally conductive fillers.
Graphite nanoplatelets (GNPs) possess super diameter/thickness ratio, can disperse uniformly in polymer matrix and contact each other easily, which are benefit for achieving a low percolation threshold.29 Furthermore, GNPs have a similar thermal conductivity value as graphene, but are much cheaper than that of graphene.30,31 Therefore, it is expected that GNPs are suitable for fabricating the polymeric composites with a relative higher thermal conductivity. Research in the K. Kalaitzidou group32 has shown that GNPs can enhance the thermal conductivity of the polypropylene (PP). The maximum thermal conductivity value measured for 25 vol% GNPs/PP composites was six times higher than that of original PP.
Polyphenylene sulfide (PPS), possesses superior chemical resistance, excellent mechanical properties & dimensional stability, high temperature resistance and low thermal expansion coefficient, and has been one of the ideal choices for the microelectronic packaging materials.33,34 S. Y. Pak35 reported a high thermal conductivity BN/MWCNT/PPS composite (λ = 1.74 W m−1 K−1). Herein, the improvement of thermal conductivity is attributed to the generation of thermally conductive networks between BN/MWCNT and PPS matrix. Additionally, S. P. Ju36 investigated the thermal conductivities of GNPs/PPS composites by experimental measurement and non-equilibrium molecular dynamics simulation. Results showed that, at the highest GNPs mass fractions of 40%, the thermal conductivity value for the injection and hot press methods were enhanced by 6 and 4 times those of the thermal conductivity of original PPS. The improvement of the thermal conductivities of the composites was not as obvious as expected previously.
To our best knowledge, a low percolation threshold can be achieved for the polymeric composites with segregated structures. The common method to fabricate the segregated structural composites is dry-mixing followed by compression molding, which can embed the thermal conductivity fillers onto the surface of polymer matrix.37–39
In our present work, the method of mechanical ball milling followed by compression molding is introduced to fabricate thermal conductivity GNPs/PPS composites with segregated structures. And surface functionalized pristine GNPs (fGNPs) by methanesulfonic acid/isopropyltrioleictitanate (MSA/NDZ-105) are proposed to further improve the thermal conductivities and mechanical properties of the GNPs/PPS composites.
Materials and methods
Materials
Pristine graphite nanosheets (GNPs), KNG-180, with diameter of 40 μm, super diameter/thickness ratio of 250, are received from Xiamen Knano Graphene Technology Co. Ltd. (Fujian, China); polyphenylene sulfide (PPS), 1.43 g cm−3, is supplied by Nanjing Deyuan Science and Technology Co. Ltd. (Jiangsu, China); methanesulfonic acid (MSA) is received from Chengdu Kelong Chemical Co. Ltd. (Sichuan, China); titanate coupling reagent of isopropyltrioleictitanate (NDZ-105), is supplied by Nanjing Shuguang Chemical Group Co., Ltd. (Jiangsu, China); sodium hydroxide (NaOH), isopropyl alcohol, tetrahydrofuran (THF) and absolute ethanol are all purchased from Tianjin Ganglong Chemical Group Co., Ltd. (Tianjin, China).Surface functionalization of pristine GNPs
Surface functionalization of pristine GNPs will hopefully ensure good dispersion of GNPs in PPS matrix, to improve the interfacial compatibility between GNPs and PPS matrix. The proposed method of MSA/NDZ-105 differs from the common oxidation process, to hardly disrupt the p conjugation and decrease the thermal conductivity of GNPs.Pristine GNPs are firstly immerged in THF and absolute ethanol for 12 h at room temperature for each step, followed by the connection of MSA (concentration of 30% by weight), NaOH (concentration of 10% by weight) and NDZ-105 molecules (more detailed introduction in the literature27). Finally, the functionalized GNPs (fGNPs) are stored at 80 °C vacuum oven. Fig. 1 shows the general process of fGNPs.40
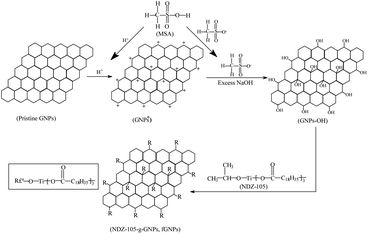 | ||
| Fig. 1 Schematic illustrating a process of as-grown GNPs transformation to functionalized GNPs (fGNPs). | ||
Preparation of the composites
GNPs and PPS are dried in vacuum oven at 70 °C for 6 h firstly. And the GNPs/PPS composites are prepared according to the following procedures: (i) mixing GNPs and PPS using ball mill machine for 24 h at room temperature, to embed the GNPs onto the surface of PPS matrix; (ii) compression molding (295 °C, 10 MPa) to fabricate the thermal conductivity GNPs/PPS composites with segregated structures.Characterization
Scanning electric microscope (SEM) morphologies of the samples are analyzed by VEGA3-LMH (TESCAN Corporation, Czech Republic); the thermo-gravimetric analyses (TGA) of samples are performed using a thermoanalyzer (STA 449F3, Netzsch Group, Germany) in the temperature range of 40–1000 °C with a heating rate of 20 K min−1 under argon atmosphere and a gas flow of 150 mL min−1; thermally conductive coefficients of the samples are measured using a Hot Disk instrument (AB Corporation, Sweden) according to standard ISO 22007-2: 2008; flexural strength and impact strength of the samples are determined using Electron Omnipotence Experiment Machine SANS-CMT5105 (Shenzhen New Sansi Corporation, China) according to standard ISO178: 2001 and standard ISO179: 2000, respectively.Results and discussion
Dispersion of pristine GNPs and fGNPs
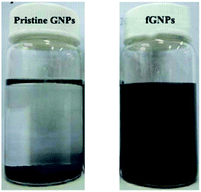
The dispersion states of pristine GNPs and fGNPs are shown in Fig. 2. The pristine GNPs are easily aggregated and precipitated at the bottom of THF solvent. However, fGNPs maintain a colloidal stability in THF solvent for more than 24 h. The reason is that NDZ-105 can provide better compatibility between GNPs and THF solvent, which can prevent the GNPs from aggregates due to Van der Waals' force, finally to provide remarkably stable suspension in THF solvent.Mechanical properties the GNPs/PPS composites
Fig. 3 shows the mass fraction of GNPs influencing on the mechanical properties of the GNPs/PPS composites.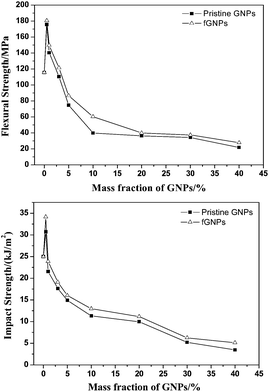 | ||
| Fig. 3 The mechanical properties of the GNPs/PPS composites. (a) Flexural strength; (b) impact strength. | ||
Both the flexural and impact strength of the composites are increased up to 0.5 wt% incorporation, but decreased with excessive addition of GNPs. The mechanical properties of the GNPs/PPS composites are maximal with 0.5 wt% addition of GNPs. Compared with original PPS (115.5 MPa of flexural strength and 24.9 kJ m−2 of impact strength), the corresponding flexural strength (180.6 MPa) and impact strength (34.2 kJ m−2) of the GNPs/PPS composites are increased by 56 percent and 37 percent, respectively. Furthermore, for a given GNPs loading, the fGNPs/PPS composites possess better mechanical properties than those of pristine GNPs/PPS composites.
Appropriate GNPs can effectively transfer stress, cause shear yield and prevent the crack propagation inside the PPS matrix. Under external forces, produced deformation can make the stress relaxation easily, finally to improve the mechanical properties of the GNPs/PPS composites. However, more interfacial defects and stress concentration points are easily introduced into the PPS with excessive addition of GNPs, finally to decrease the mechanical properties of the composites.
After surface functionalization of GNPs, the inner defects in the composites are decreased obviously (Fig. 4). It reveals that fGNPs have better interfacial compatibility with PPS matrix, which is benefit for decreasing the inner defects, finally to increase the mechanical properties of the composites.
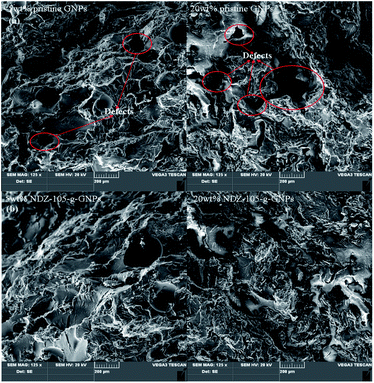 | ||
| Fig. 4 SEM morphologies of the composites (a) pristine GNPs/PPS composites; (b) fGNPs/PPS composites. | ||
Thermal conductivities the composites
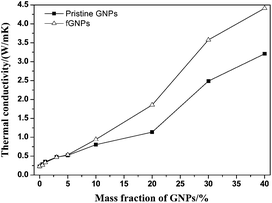
The mass fraction of GNPs influencing on the thermal conductivities of the GNPs/PPS composites is shown in Fig. 5.The thermal conductivities of the GNPs/PPS composites are increased with the increasing mass fraction of GNPs. For a given GNPs loading, the fGNPs/PPS composites possess better thermal conductivities than those of pristine GNPs/PPS composites. The thermally conductive coefficient of the fGNPs/PPS composites with 40 wt% fGNPs is greatly improved to 4.414 W m−1 K−1, 19 times higher than that of the original PPS (0.226 W m−1 K−1).
GNPs with low mass fraction have weak interaction each other to present a relatively little increasing thermal conductivities. With the increasing addition of GNPs, the interconnected function between GNPs and GNPs is improved obviously, and the probabilities of thermally conductive networks are increased, thus the thermal conductivities of the GNPs/PPS composites are improved obviously.
Furthermore, the GNPs/PPS composites exhibit a rapid improvement of thermal conductivities in the range of 10–40 wt% addition of GNPs. The reason is that GNPs with super diameter/thickness ratio can achieve a lower thermal percolation threshold. In addition, our proposed method can fabricate the GNPs/PPS composites with segregated structures. GNPs are located on the interface of PPS particles instead of being randomly distributed in the PPS matrix. Finally a thermally conductive network can be generated. Therefore, the ultimate thermal conductivities of the GNPs/PPS composites are increased obviously.
After the surface functionalization of GNPs, the dispersion of fGNPs in the PPS matrix is improved, and especially the interfacial compatibility between fGNPs and PPS matrix is increased. Thus the interfacial thermal resistance between fGNPs and PPS matrix is reduced effectively, which is in favor of the phonon transport, finally to increase the thermal conductivities of the fGNPs/PPS composites.
Fig. 6 shows the SEM morphologies of the fGNPs/PPS composites. It can be seen that a small amount of fGNPs are dispersed uniformly in the PPS matrix, and there is some connectivity between PPS particles in small regions (Fig. 6b). With the further increasing addition of fGNPs, fGNPs can pack tightly to contact each other, and the thermally conductive networks can be generated (Fig. 6c–f).
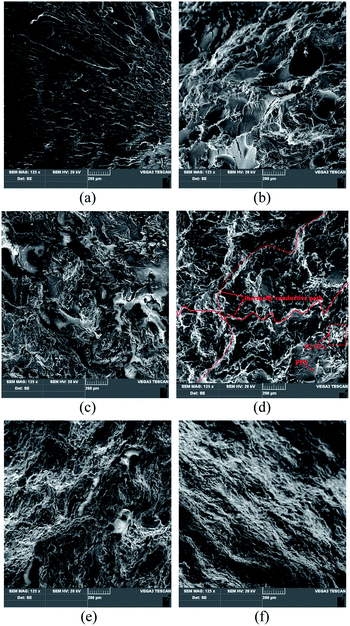 | ||
| Fig. 6 SEM morphologies of the fGNPs/PPS composites. (a) Original PPS; (b) fGNPs/PPS (5/95); (c) fGNPs/PPS (10/90); (d) fGNPs/PPS (20/80); (e) fGNPs/PPS (30/70); (f) fGNPs/PPS (40/60). | ||
| THeat-resistance index = 0.49 × [T5 + 0.6 × (T30 − T5)] | (1) |
| Samples | Weight loss/% | Heat-resistance indexa/°C | ω/% | |
|---|---|---|---|---|
| 5% | 30% | |||
| a The sample's heat-resistance index was calculated by eqn (1). | ||||
| Original PPS | 509 | 566 | 266 | 45.6 |
| fGNPs/PPS (5/95) | 511 | 571 | 268 | 48.9 |
| fGNPs/PPS (10/90) | 514 | 574 | 270 | 51.2 |
| fGNPs/PPS (20/80) | 515 | 579 | 271 | 56.0 |
| fGNPs/PPS (30/70) | 516 | 590 | 275 | 61.6 |
| fGNPs/PPS (40/60) | 521 | 610 | 281 | 63.8 |
Conclusion
The thermal conductivities of the GNPs/PPS composites are improved with the increasing mass fraction of GNPs, and the thermally conductive coefficient of the fGNPs/PPS composite with 40 wt% fGNPs is greatly improved to 4.414 W m−1 K−1, about 19 times higher than that of original PPS (0.226 W m−1 K−1).For a given GNPs loading, the surface functionalization of pristine GNPs by MSA/NDZ-105 results in the composites improving thermal conductivities by minimizing the interfacial thermal resistance, and to increase the mechanical properties by improving the uniform dispersion of the fGNPs in the PPS matrix.
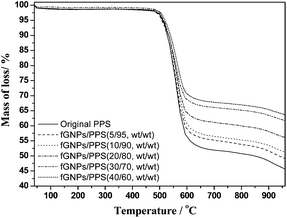
TGA analyses indicate that the thermal stabilities of the fGNPs/PPS composites are increased with the increasing addition of fGNPs. SEM observations reveal that the thermally conductive networks have formed in the fGNPs/PPS composites, when the mass fraction values of GNPs exceed the thermally conductive percolation threshold.
Acknowledgements
The authors are grateful for the support and funding from National Natural Science Foundation of China (no. 81100783), Higher School the Special Research Fund for the Doctoral Program Project (no. 20116102120044), Shaanxi National Science Foundation of Shaanxi Province (no. 2014JQ6203), Open Fund from National Defense Science and Technology Key Laboratory (no. 2013AFDL007) and Fund for basic research from the Northwestern Polytechnical University (no. JCY20130143).References
- K. M. F. Shahil and A. A. Balandin, Nano Lett., 2012, 12, 861–867 CrossRef CAS PubMed.
- J. Bouchard, A. Cayla, E. Devaux and C. Campagne, Compos. Sci. Technol., 2013, 86, 177–184 CrossRef CAS PubMed.
- M. D. Losego, M. E. Grady, N. R. Sottos, D. G. Cahill and P. V. Braun, Nat. Mater., 2012, 11, 502–506 CrossRef CAS PubMed.
- D. S. Muratov, D. V. Kuznetsov, I. A. Il'inykh, I. N. Mazov, A. A. Stepashkin and V. V. Tcherdyntsev, J. Alloys Compd., 2014, 586, S451–S454 CrossRef CAS PubMed.
- T. Luo and J. R. Lloyd, Adv. Funct. Mater., 2012, 22, 2495–2502 CrossRef CAS.
- X. P. Huang, G. Q. Liu and X. W. Wang, Adv. Mater., 2012, 24, 1482–1486 CrossRef CAS PubMed.
- J. K. Yang, Y. Yang, S. W. Waltermire, X. X. Wu, H. T. Zhang and T. Gutu, Nat. Nanotechnol., 2012, 7, 91–95 CrossRef CAS PubMed.
- Q. Liang, X. Yao, W. Wang, Y. Liu and C. P. Wong, ACS Nano, 2011, 5, 2392 CrossRef CAS PubMed.
- S. H. Song, K. H. Park, B. H. Kim, Y. W. Choi, G. H. Jun and D. J. Lee, Adv. Mater., 2013, 25, 732–737 CrossRef CAS PubMed.
- I. H. Tseng, J. C. Chang, S. L. Huang and M. H. Tsai, Polym. Int., 2012, 62, 827–835 CrossRef.
- S. R. Wang, R. Liang, B. Wang and C. Zhang, Carbon, 2009, 47, 53–57 CrossRef CAS PubMed.
- S. Yang, C. M. Ma, C. Teng, Y. Huang, S. Liao and Y. Huang, Carbon, 2010, 48, 592–603 CrossRef CAS PubMed.
- L. M. Gao, T. W. Chou, E. T. Thostenson, A. Godara, Z. G. Zhang and L. Mezzo, Carbon, 2009, 47, 1958–1968 CrossRef PubMed.
- I. D. Rosca and S. V. Hoa, Carbon, 2012, 48, 2644–2673 Search PubMed.
- X. Y. Huang, C. Y. Zhi, P. K. Jiang, D. Golberg, Y. Bando and T. Tanaka, Adv. Mater., 2013, 23, 1824–1831 CAS.
- H. He, R. L. Fu, Y. Shen, Y. C. Han and X. F. Song, Compos. Sci. Technol., 2007, 67, 2493–2499 CrossRef CAS PubMed.
- W. Y. Zhou, C. F. Wang, T. Ai, K. Wu, F. J. Zhao and H. Z. Gu, Composites, Part A, 2009, 40, 830–836 CrossRef PubMed.
- T. L. Zhou, X. Wang, X. H. Liu and D. S. Xiong, Carbon, 2010, 48, 1171–1176 CrossRef CAS PubMed.
- K. Yang and M. Y. Gu, Composites, Part A, 2012, 41, 215–221 CrossRef PubMed.
- H. M. Tu and L. Ye, Polym. Adv. Technol., 2009, 20, 21–27 CrossRef CAS.
- S. Ganguli, A. K. Roy and D. P. Anderson, Carbon, 2008, 46, 806–817 CrossRef CAS PubMed.
- X. W. Zhao and L. J. Ye, Appl. Polym. Sci., 2009, 111, 759–767 CAS.
- Y. C. Zhang, K. Dai, J. H. Tang, X. Ji and Z. M. Li, Mater. Lett., 2012, 64, 1430–1432 CrossRef PubMed.
- X. Y. Hao, G. S. Gai, Y. F. Yang, Y. H. Zhang and C. W. Nan, Mater. Chem. Phys., 2008, 109, 15–19 CrossRef CAS PubMed.
- J. W. Gu, Q. Y. Zhang, J. Dang, C. J. Yin and S. J. Chen, J. Appl. Polym. Sci., 2012, 124, 132–137 CrossRef CAS.
- J. W. Gu, Q. Y. Zhang, J. Dang and C. Xie, Polym. Adv. Technol., 2012, 23, 1025–1028 CrossRef CAS.
- J. W. Gu, Q. Y. Zhang, J. Dang, J. P. Zhang and Z. Y. Yang, Polym. Eng. Sci., 2009, 49, 1030–1034 CAS.
- J. W. Gu, Q. Y. Zhang, J. Dang, J. P. Zhang and S. J. Chen, Polym. Bull., 2009, 62, 689–697 CrossRef CAS.
- L. Y. Zhang and G. H. Chen, Mater. Rev., 2011, 25, 85–88 CAS.
- R. R. Nair, P. Blake, A. N. Grigorenko, K. S. Novoselov, T. J. Booth and T. Stauber, Science, 2008, 320, 1308 CrossRef CAS PubMed.
- L. H. Liu and M. D. Yan, J. Mater. Chem., 2011, 21, 3273–3276 RSC.
- K. Kalaitzidou, H. Fukushima and L. T. Drzal, Carbon, 2007, 45, 1446–1452 CrossRef CAS PubMed.
- J. Yang, Polyphenylene sulfide resin and its application, Chemical Industry Press, Beijing, 2006 Search PubMed.
- A. M. Diez-Pascual, J. W. Guan, B. Simard and M. A. Gómez-Fatou, Composites, Part A, 2012, 43, 1007–1015 CrossRef CAS PubMed.
- S. Y. Pak, H. M. Kim, S. Y. Kim and J. R. Youn, Carbon, 2012, 50, 4830–4838 CrossRef CAS PubMed.
- S. P. Ju, T. J. Haung, C. H. Liao and J. W. Chang, Polymer, 2013, 54, 4702–4709 CrossRef CAS PubMed.
- C. Zhang, C. A. Ma, P. Wang and M. Sumita, Carbon, 2005, 43, 2544–2553 CrossRef CAS PubMed.
- R. Q. Ou, S. Gupta, C. A. Parker and R. A. Gerhardt, J. Phys. Chem. B, 2006, 110, 22365–22373 CrossRef CAS PubMed.
- J. F. Gao, Z. M. Li, Q. J. Meng and Q. Yang, Mater. Lett., 2008, 62, 3530–3532 CrossRef CAS PubMed.
- J. W. Gu, C. Xie, H. L. Li, J. Dang, W. C. Geng and Q. Y. Zhang, Polym. Compos., 2014 DOI:10.1002/pc.22756.
| This journal is © The Royal Society of Chemistry 2014 |