Enhanced photocatalytic degradation of congo red by solvothermally synthesized CuInSe2–ZnO nanocomposites†
Minoo Bagheri,
Ali Reza Mahjoub* and
Behnam Mehri
Department of Chemistry, Tarbiat Modares University, Tehran, Iran. E-mail: mahjouba@modares.ac.ir; Fax: +98 21 82883455; Tel: +98 21 82883442
First published on 30th April 2014
Abstract
Efficient photocatalysts of CuInSe2–ZnO nanocomposites were prepared via a solvothermal method using a mixed solvent of ethylenediamine and ethanol (volumetric ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The products were characterized by SEM, EDX, XRD, PL, BET surface area, PZC and DRS UV-vis techniques and used for photo-degradation of congo red. The characterization results showed that crystallite size, BET surface area and optical absorption of the samples varied significantly with the addition of CuInSe2 to ZnO. The nanocomposites show absorption edges in the ultraviolet and visible regions depending on their CuInSe2 content. The optical band gap values of these nanocomposites were calculated to be about 3.37–2.1 eV, which show a red shift from that of pure ZnO. These red shifts indicate the incorporation of CuInSe2 in the zinc oxide lattice. In the investigation of the photocatalytic activity of the samples, the effects of the experimental parameters including pH, congo red concentration, CuInSe2 content and irradiation sources of UV and visible light have also been studied. Addition of CuInSe2 was effective in improving the photocatalytic activity remarkably. The highest percentage removals of 99.8% and 80.3% are observed for the photocatalyst containing 10 wt% CuInSe2 after 90 and 120 min under UV and visible irradiation, respectively. Also, a possible removal mechanism of the samples is proposed. It could be considered as a promising photocatalyst for dye degradation.
1). The products were characterized by SEM, EDX, XRD, PL, BET surface area, PZC and DRS UV-vis techniques and used for photo-degradation of congo red. The characterization results showed that crystallite size, BET surface area and optical absorption of the samples varied significantly with the addition of CuInSe2 to ZnO. The nanocomposites show absorption edges in the ultraviolet and visible regions depending on their CuInSe2 content. The optical band gap values of these nanocomposites were calculated to be about 3.37–2.1 eV, which show a red shift from that of pure ZnO. These red shifts indicate the incorporation of CuInSe2 in the zinc oxide lattice. In the investigation of the photocatalytic activity of the samples, the effects of the experimental parameters including pH, congo red concentration, CuInSe2 content and irradiation sources of UV and visible light have also been studied. Addition of CuInSe2 was effective in improving the photocatalytic activity remarkably. The highest percentage removals of 99.8% and 80.3% are observed for the photocatalyst containing 10 wt% CuInSe2 after 90 and 120 min under UV and visible irradiation, respectively. Also, a possible removal mechanism of the samples is proposed. It could be considered as a promising photocatalyst for dye degradation.
Introduction
Currently, there is growing interest in the development of new techniques in the field of environmental protection due to the global pollution problems.1 Industrial wastes have caused too much pollution for the environment and further cause health problems for human beings.2,3 Approximately 10–15% of total world production of dye is lost during the production process and is released in the textile effluent.4 The usage of synthetic dyes such as azo-dyes especially in the textile industries and the discharge of the industrial wastes containing these compounds into the aquatic systems have continued to increase. It is known that azo dyes are highly toxic and even carcinogenic to animals and human beings and they are not readily degradable and could create dangerous byproducts through oxidation, hydrolysis, or other chemical reactions occurring in the wastewater stream.5 Congo red (CR) is a typical and the first anionic synthetic dye which has two azo (–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–) chromophores. Like other azo dyes, this compound is very stable because of its complex aromatic structure, hence it is not easily degradable.6
N–) chromophores. Like other azo dyes, this compound is very stable because of its complex aromatic structure, hence it is not easily degradable.6
Several traditional physical and chemical methods have been used for the removal of dye pollutants. However, these methods only succeed in transferring organic pollutants from water to other phases. Moreover, secondary pollution could entail additional and costly solid-waste treatment.1,7
In the two last decades, heterogeneous photocatalysis has received much attention as a promising advanced oxidation process for its capability to completely mineralize recalcitrant contaminants in water or air, which cannot be effectively removed by conventional methods.5–8
Many studies have been devoted to the synthesis of metal oxide semiconductors with special properties that have potential applications in electronics, optics, thermoelectronics, photoelectronics and photocatalysis. It is interesting to note that various properties could be observed depending on their metal ions, morphology, size and structure.9,10
ZnO-based semiconductors with a wide band gap (3.37 eV) have been identified as active photocatalysts for organic pollutants in gaseous or aqueous phases due to their non-toxicity, photochemical stability, low price, abundance in nature and being environmentally friendly.11–13 In comparison with TiO2, ZnO is a better alternative because of the numerous point defects mainly from oxygen vacancies, higher production of hydroxyl ions and higher photoactivity (by a factor of 2–3) in both UV and sunlight irradiation for the decontamination of water.4 Also, ZnO is an interesting example of materials having the capability of low temperature growth with many different kinds of morphologies including wires, rods, tubes, particles and flower shape at nano scale.14–18 Among many kinds of morphologies for ZnO, nanoparticles provide better characteristics due to larger surface area and the ability to be suspended in a solution.4
It is desirable that photocatalysts such as ZnO absorb both UV and visible lights, especially since only a small fraction of the solar spectrum (5%) is UV while visible light accounts for 45% of the solar radiation energy.12 Thus, various modifications are of considerable interest to enhance the photocatalytic efficiency of metal oxides.19–22
Chalcopyrite ternary copper indium diselenide (CuInSe2) with low band gap (∼1.00 eV),23 which is regarded as a promising material specially for photovoltaic applications due to its high absorption coefficient, optimal band gap energy, good radiation stability and low toxicity,24 has attracted much attention over the last two decades.25 To the best of our knowledge there have been few reports about photocatalytic activity of ternary semiconductors such as CuInSe2.26
In this study, photo-degradation activity of CuInSe2–ZnO nanocomposites, containing various percentages of CuInSe2 synthesized via solvothermal method using three solvents of ethylenediamine, diethylamine and a mixed solvent of ethylenediamine and ethanol (volumetric ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), in presence of CR is investigated. The photocatalytic activity of the samples significantly changes with their CuInSe2 content. Furthermore, influence of different factors such as pH and congo red concentration and irradiation source has also been studied.
1), in presence of CR is investigated. The photocatalytic activity of the samples significantly changes with their CuInSe2 content. Furthermore, influence of different factors such as pH and congo red concentration and irradiation source has also been studied.
Experimental
Reagents and chemicals
Analytical grade of copper(II) chloride dehydrate (CuCl2·2H2O, Merck), indium(III) chloride tetrahydrate (InCl3·4(H2O), Aldrich), selenium powder (Se, Merck), zinc chloride dehydrate (ZnCl2·2H2O, Merck), ethylenediamine (C2H4(NH2)2, Merck), anhydrous diethylamine (C4H11N, Merck) and absolute ethanol were purchased and used without further purification. CR (purity 99%, Aldrich) has been used as received.Synthesis of materials
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1).
1).CuInSe2 obtained using ethylenediamine solvent was synthesized according to the procedure reported in the literature.1 Also, synthesis of CuInSe2 using diethylamine solvent was done in a similar procedure, the only difference being that the autoclave was maintained at 180 °C oven for 48 h. While for synthesis of CuInSe2 with a mixed solvent of ethylenediamine and ethanol (volumetric ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) another procedure reported in the literature3 was used in which capacity of autoclave was 50 mL instead of 25 mL.
1) another procedure reported in the literature3 was used in which capacity of autoclave was 50 mL instead of 25 mL.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) were stirred in a teflon lined stainless steel autoclave of 50 mL capacity for 30 min. After sealing, the autoclave was heated at 200 °C for 24 h and then cooled to room temperature. The white precipitate was collected by centrifugation and then washed several times with double distilled water and ethanol, and then dried in an oven at 60 °C under vacuum for 6 h.
1) were stirred in a teflon lined stainless steel autoclave of 50 mL capacity for 30 min. After sealing, the autoclave was heated at 200 °C for 24 h and then cooled to room temperature. The white precipitate was collected by centrifugation and then washed several times with double distilled water and ethanol, and then dried in an oven at 60 °C under vacuum for 6 h.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) under vigorous stirring at room temperature (RT) for 10 minutes. An appropriate amount of zinc chloride dihydrate was separately stirred with 25 mL of the same mixed solvent. Both mixtures were taken in a stainless steel autoclave (250 mL), heated slowly to 185 °C and maintained at that temperature for 36 h. The light/dark gray precipitate thus formed was separated from excess solvent by centrifugation and washed thoroughly with double distilled water and ethanol and dried at 60 °C under vacuum for 6 h. The samples were denoted as ZC-x, where “x” stands for percentage of CuInSe2.
1) under vigorous stirring at room temperature (RT) for 10 minutes. An appropriate amount of zinc chloride dihydrate was separately stirred with 25 mL of the same mixed solvent. Both mixtures were taken in a stainless steel autoclave (250 mL), heated slowly to 185 °C and maintained at that temperature for 36 h. The light/dark gray precipitate thus formed was separated from excess solvent by centrifugation and washed thoroughly with double distilled water and ethanol and dried at 60 °C under vacuum for 6 h. The samples were denoted as ZC-x, where “x” stands for percentage of CuInSe2.Characterization
The size and morphology of the CuInSe2–ZnO nanocomposites were observed by scanning electron microscopy (SEM) at an acceleration voltage of 15.0 kV by a Holland Philips XL30 microscope instrument. Energy Dispersed X-ray analysis (EDX) was performed by the same microscope to investigate the elemental composition of the samples. The structure and phase purity of the samples were identified by wide-angle powder X-ray diffraction (XRD) on Holland Philips X'pert diffractometer X-pert using Cu Kα radiation (λ = 1.5418 Å). Specific surface area (SSA) of the nanocomposites was determined by nitrogen adsorption using CHEM-BET 3000 instrument using BET equation where the samples were degassed for 30 min at 300 °C at 1 atm. The photoluminescence (PL) spectra were measured using Perkin Elmer LS-5 luminescence spectrometer with a wavelength of 254 nm as the excitation source at room temperature. UV-vis diffuse reflectance spectra were recorded on an Avantes, reflection probes fcr-7uv400 using BaSO4 as a reference. Surface charge densities – point of zero charge (PZC) – of the sample were measured using Zeta Pals Brookhaven U.S.A instrument.Evaluation of photocatalytic activity
The photocatalytic activity of CuInSe2–ZnO nanocomposites was evaluated via a probe reaction on the degradation of CR in aqueous medium under UV and/or visible light irradiation.The photocatalysis of CR was carried out in a cylindrical quartz UV-reactor with an effective volume of 100 mL. The UV and visible illumination was provided by a 30 W lamp (UV-C, λ = 253.7 nm, photon provides 4.89 eV, manufactured by Philips, Holland) and/or a 500 W lamp (high-pressure mercury-vapor lamp, 400 W and λ = 546.8 nm, Yaming Company, Shanghai), cooled by water flow and pH was adjusted using dilute hydrochloric acid or sodium hydroxide. 50 mL of CR dye solution was implemented to the photoreactor, and 25 mg of CuInSe2–ZnO photocatalyst was also added to the reactor. An air diffuser (air pump, flow: 4.5 L min−1) was placed at the bottom of the reactor to uniformly disperse air into the solution while the mixture was stirred. The suspension was sonicated for 5 min and then stirred in the dark for 30–45 min (depending on the type of samples and their darkness times found based on the absorption experiments), to ensure an adsorption/desorption equilibrium on the semiconductor surface prior to irradiation. Perpendicular UV and/or visible irradiation with a distance of about 15 cm between the light source and the reaction mixture were applied. Samples for analyses were taken from the reaction suspension at specified reaction times and immediately centrifuged at 6000 rpm for 10 min to remove the particles and were further analyzed by monitoring the absorbance at 498 nm using UV-Vis spectrophotometer (Shimadzu UV 2100). The concentration of dye in each degraded sample was determined at λmax = 498 nm, using a calibration curve. The percentage removal of CR is calculated as follows:
| %Removal = (Ci − Ct)/Ci × 100 | (1) |
The chemical oxygen demand (COD) test is widely used as an effective technique to measure the organic strength of wastewater. This test allows the measurement of waste in terms of the total quantity of oxygen required for the oxidation of organic matter to CO2 and water. The open reflux method was applied for COD determination.27
The photocatalyst stability tests were performed in the same way as the photocatalytic activity tests but they were repeated four times.
Results and discussion
Characterization of CuInSe2–ZnO nanocomposites
Elemental analyses of the CuInSe2–ZnO samples determined by EDX are presented in Fig. S1 and Table S1† which are in good agreement with the nominal composition of the samples. The BET specific surface area (SSA) of the samples containing various proportions of CuInSe2 is presented in Table 1. Generally, the surface area of the samples increases upon adding more CuInSe2 to zinc oxide. A sharp increase in SSA is observed for the sample containing 10 wt% CuInSe2. Among the obtained samples the ZC-50 shows the highest SSA of 37 m2 g−1.| Samples | dXRD | Crystallinity (%) | BET area (m2 g−1) | dBET | Band gap (eV) |
|---|---|---|---|---|---|
| CISe | 5.7 | 90.3 | 1.3 | 7.3 | — |
| Z | 40 | 92.9 | 16.3 | 45.4 | 3.37 |
| ZC-3 | — | — | 23 | 46.4 | 3.2 |
| ZC-5 | 37.3 | 85.64 | 25.3 | 42.2 | 3.1 |
| ZC-10 | 30.8 | 88.97 | 31.5 | 33.8 | 2.6 |
| ZC-25 | 25.6 | 81.58 | 34.5 | 30.8 | 2.4 |
| ZC-50 | 23.2 | 80.12 | 37.1 | 28.5 | 2.1 |
The PL spectra of pure ZnO and ZC-10, containing 10 wt% CuInSe2, samples are shown in Fig. 1. The PL spectrum of pure ZnO consists of a broad band centered at around 410 nm and a sharp band centered at around 500 nm with a shoulder at 489 nm. The UV emission resulted from the recombination of free excitons and the green emission is related to the recombination of a photo-generated hole with a singly ionized charge state of specific defect specially oxygen vacancies.28,29 The PL spectrum of ZC-10 is significantly quenched in the UV range 338–437 nm, indicating a lower rate of recombination between the photo-generated hole and electrons on the surface of the pure ZnO.30 It should be noted that the green emission for ZC-10 sample is more intense than that for the pure sample.31,32 We suppose that an increase in emission intensity is an indication of an increase in concentration of the oxygen vacancies.33
Fig. S2† shows that pure CuInSe2 can be obtained only using the mixed solvent while by-products remain with CuInSe2, when using the two other solvents. Some impurities such as In2Se3 were detected in the XRD patterns. According to the literature,34 amine can activate selenium as Se2− by a nucleophilic attack in the solvothermal process together with In2Se3 precipitate as follows:
| 2InCl3 + 3Se2− → In2Se3 + 6Cl− | (2) |
These results show that solvents play a major role in the formation of the chalcopyrite CuInSe2. Only solvents with suitable reducing or coordination ability such as alkylamines can reduce Cu2+ ions and form activated selenium.
As mentioned in the literature35 mixed solvent of ethylenediamine–ethanol appeared to be the optimal reaction medium to build up CuInSe2 samples. In2Se3 intermediate has low solubility in ethylenediamine. In presence of mixed solvent with reduced polarity, the solubility of In2Se3 increases. As a result, the site of nucleation becomes bigger and fast individual nuclei growth suppresses the anisotropic growth of the particles, leading to the formation of CuInSe2. Furthermore, substitution of toxic ethylenediamine to ethanol leads to a decrease in toxicity of the reaction medium.
As presented in Fig. 2, for these samples containing 0–5 wt% CuInSe2, Bragg diffraction peaks in the range of 2θ = 20–80°, show the typical patterns of a wurtzite structure of ZnO which is in good agreement with the reported data by JCPDS card no.43-1012, with lattice parameters a = b = 3.2 and c = 5.2 Å. The strongest peak for hexagonal was observed at 2θ = 33.2°. No peak in the XRD patterns of the samples, with 0–5 wt% CuInSe2, can be assigned to any known phase of CuInSe2, probably because of law content of CuInSe2 and/or small crystallite sizes not detected in XRD patterns. For other samples containing 10–50 wt% CuInSe2 both of the ternary compound with tetragonal structure CuInSe2 and wurtzite ZnO structure are observed. In the case of ZC-10 sample, the peaks of CuInSe2 are weak as compared to those of ZnO. However, in ZC-25 and ZC-50 samples, the peaks of CuInSe2 are strong as compared to the ZC-10 peaks.
The crystallinity of the samples, determined by ratio of crystalline area to total area of each XRD pattern. The crystallinity tends to decrease for up to 5 wt% CuInSe2, while an increasing in the sample with 10 wt% CuInSe2, and decreasing again for samples of above 10 wt% CuInSe2.
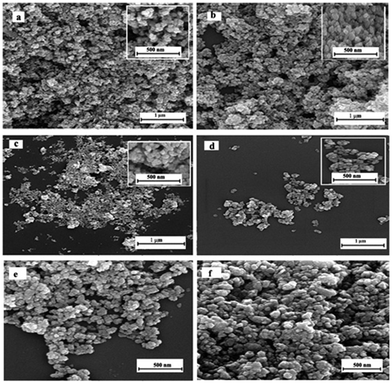
Morphologies of CuInSe2–ZnO samples are recorded using scanning electron microscopy shown in Fig. 3a–f for Z, ZC-3, ZC-5, ZC-10, ZC-25 and ZC-50. For pure ZnO the morphology is nanoparticle. Morphology difference between samples is insignificant. The addition of CuInSe2 decreases the particle size so the only difference is particle size of samples. These observations confirm that CuInSe2 may control the particle growth and thus influence particle size. These results are also in accordance with the results of BET.
UV-vis diffuse reflectance spectra of Z and ZC-10 samples are shown in Fig. 4. The optical band gaps of samples are estimated by extrapolating the linear portion of the square of absorption coefficient against photon energy using the equation:
| (αhν)2 = B(hν − Eg) | (3) |
 | ||
| Fig. 4 (a) Optical absorption spectra of Z and ZC-10 samples and (b) (αhν)2 versus photon energy plots of the mentioned samples. | ||
This is due to the absorption of light caused by the excitation of electrons from the valence band to the conduction band of zinc oxide.37 The optical band gap of samples also decreases with an increase their CuInSe2 content. This substantiates the dramatic effect of CuInSe2 on decreasing the band gap (Table 1). Thus the DRS UV-vis spectra confirmed the framework incorporation of CuInSe2 in ZnO lattice. In other words, addition of CuInSe2 can form new states level into the ZnO. The decrement of the band gap is at about 1.3 eV from 0 to 50 wt% CuInSe2, which could narrow down the band gap of ZnO by impurity energy, much smaller than that of pure ZnO.
Photocatalytic activity
Therefore, all further solutions with various wt% of CuInSe2 were adjusted at optimum pH of 5 to improve adsorption.
The surface charge data plotted in the form of δ0–pH curves for ZC-10 sample are presented in Fig. 5b. In the pH limit 5–10, there is a linear decline in surface charge with pH which is then followed by a sharp decrease at about pH 10. Also, the surface charge densities, point of zero charge (PZC), for pure ZnO are at about pH 8.9 (ref. 38) which decrease with an increase in CuInSe2 content of sample. The PZC values determined from the δ0–pH curves are 5.5 for ZC-10 sample.
On the other hand, addition of CuInSe2 resulted in a more acidic ZnO surface.
With regard to PZC of photocatalyst surface and the nature of dye (cationic, anionic or neutral), solution pH is a significant effect on adsorption efficiency dyes on adsorbent. In pH < pzc, the adsorbent surface is positively charged and due to the anionic nature of CR, electrostatic attractions result in dye adsorption and improvement of removal efficiency of CR, while in pH > pzc, the surface is negatively charged39 and following enhancement in electrostatic repulsion, removal efficiency of CR decreases. Another explanation regarding the behavior of acidic pH is the existence of hydrogen bonds between photocatalyst's hydroxyl groups and sulfonate or amine sites of dye molecules. As pH of the CR solution increased, an extreme decrease in adsorption took place. Generally at higher pHs, the anion OH− can compete with anionic sites of CR dye which is adsorbed onto positive charges of photocatalyst. This leads to blocking of activated sites.40
| [CR] (ppm) | Removal% | Tdark (min) | Tremoval (min) | Removal% | |||
|---|---|---|---|---|---|---|---|
| (No catalyst) | Z | ZC-10 | Z | ZC-10 | Z | ZC-10 | |
| 5 | 0 | 20 | 10 | 120 | — | 99.7 | 99.9 (Ads.) |
| 10 | 0 | 20 | 20 | 120 | — | 89.4 | 99.9 (Ads.) |
| 20 | 0 | 25 | 30 | 180 | — | 79.8 | 99.9 (Ads.) |
| 30 | 0 | 30 | 30 | 180 | 90 | 43.7 | 99.8 |
| 40 | 0.05 | 30 | 30 | 180 | 120 | 40.3 | 70.2 |
| 50 | 0.05 | 30 | 30 | 180 | 120 | 37.3 | 63.9 |
One possible explanation of such circumstances is that as initial concentration increases, more and more organic substances are adsorbed on the surface of sample. Scarcity of active sites in the system causes little adsorption of hydroxyl ions which in turn leads to a decrease in generation of hydroxyl radicals. Further, as the concentration of dye increases, the photons get intercepted before they can reach the catalyst surface, thus the photon adsorption by the catalyst decreases. Consequently, percentage removal is reduced.1
Therefore, the photocatalytic tests for all samples containing various wt% of CuInSe2 were carried out in presence of 30 ppm of CR.
The photocatalytic efficiency of ZC-10, ZC-25 and ZC-50 nanocomposites in presence of 30 ppm CR under visible light irradiation for 2 h is investigated. As shown in Table S2,† ZC-10 sample has the best efficiency among the two other samples at about 80.3% for 2 h. As mentioned above, the highest percentage removals of 99.8% and 80.3% are observed for the photocatalyst containing 10 wt% CuInSe2 after 90 under UV and 120 min under visible irradiations, respectively.
The photocatalytic performance could be attributed to the high surface area, the value of band gap and the difference in the rate of recombination, the crystallinity of a photocatalyst, the morphology, low degree of agglomeration, the type of polymorph and lattice structure, defects specially oxygen vacancies and adsorptive affinity.41,42 With regards to the PL, BET and XRD results, on one hand, the greater concentration of oxygen vacancies cause a greater proper value for SSA and on the other hand, the presence of two different phases (ZnO and some new weak reflections of CuInSe2 in its structure) with a proper band gap leads to the lower recombination rate. As a result ZC-10 shows the highest activity compared to other samples.
Fig. 6a presents the COD values for the best photocatalyst sample of “ZC-10”. As shown in the figure, in each case, the COD value of the initial color solution initially increases due to cleavage of rings in dye molecule and then significantly decreases after 72 h, indicating the high potential of the CuInSe2–ZnO photodegradation process for the removal of CR from wastewater. The photo-degradation efficiency is found to be 70.6%. This result confirms that ZC-10 sample is a good candidate for photo-degradation of CR in wastewater. Also, ZC-10 photocatalyst is very stable during four repeated experiments (Fig. 6b). The XRD pattern of ZC-10 photocatalyst before and after repeating the reaction four times is shown in Fig. 6c which clearly indicates that the structure remained the same and no adsorption was observed.
ZnO is an n-type semiconductor while CuInSe2 is a p-type one. Hence Fermi energy level is distinct of each other. For ZnO, Fermi energy level is located to the conduction band, while Fermi energy levels of CuInSe2 lie close to valence band. When ZnO and CuInSe2 are in contact with each other, their Fermi energy levels reach equilibration. Fermi energy levels of ZnO and CuInSe2 tend to shift downward and upward, respectively. The resulting photo-generated electrons transfer from the conduction band of CuInSe2 to the conduction band of ZnO, whereas the photo-generated holes transfer in the opposite direction along with valence bands.26 CuInSe2–ZnO nanocomposite prevents from the recombination of the electron–hole pairs, thus resulting in more holes can participate in the photooxidation process.
Conclusions
In summary, we synthesized UV and visible light sensitive photocatalysts of CuInSe2–ZnO nanocomposites with various proportions of CuInSe2 by a simple solvothermal method using a mixed solvent of ethylenediamine and ethanol (volumetric ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The crystallite size, BET surface area and optical absorption of the nanostructures are strongly affected by addition of CuInSe2 to ZnO. The addition of CuInSe2 to ZnO semiconductor gives lower band gap values with a red shift for nanocomposites compared to pure ZnO. When treated with 30 ppm of congo red, 10 wt% CuInSe2–ZnO nanocomposite exhibits the highest photo-degradation due to proper values of SSA and band gap that cause greater concentration of oxygen vacancies and a lower recombination rate, which is promising for applications in water detoxification.
1). The crystallite size, BET surface area and optical absorption of the nanostructures are strongly affected by addition of CuInSe2 to ZnO. The addition of CuInSe2 to ZnO semiconductor gives lower band gap values with a red shift for nanocomposites compared to pure ZnO. When treated with 30 ppm of congo red, 10 wt% CuInSe2–ZnO nanocomposite exhibits the highest photo-degradation due to proper values of SSA and band gap that cause greater concentration of oxygen vacancies and a lower recombination rate, which is promising for applications in water detoxification.
Acknowledgements
Financial support of this work by Tarbiat Modares University is gratefully acknowledged.Notes and references
- S. Erdemoğlu, S. K. Aksu, F. Sayılkan, B. İzgi, M. Asiltürk, H. Sayılkan, F. Frimmel and Ş. Güçer, J. Hazard. Mater., 2008, 155, 469–476 CrossRef PubMed.
- J. Wang, Y. Jiang, Z. Zhang, G. Zhao, G. Zhang, T. Ma and W. Sun, Desalination, 2007, 216, 196–208 CrossRef CAS PubMed.
- F. Zhang, Y. Liu, Y. Cai, H. Li, X. Cai, I. Djerdj and Y. Wang, Powder Technol., 2013, 235, 121–125 CrossRef CAS PubMed.
- I. Udom, M. K. Ram, E. K. Stefanakos, A. F. Hepp and D. Y. Goswami, Mater. Sci. Semicond. Process., 2013, 16, 2070–2083 CrossRef CAS PubMed.
- V. A. Sakkas, M. A. Islam, C. Stalikas and T. A. Albanis, J. Hazard. Mater., 2010, 175, 33–44 CrossRef CAS PubMed.
- R. Ramakrishnan, S. Kalaivani, J. Amala Infant Joice and T. Sivakumar, Appl. Surf. Sci., 2012, 258, 2515–2521 CrossRef CAS PubMed.
- H. Zhu, R. Jiang, L. Xiao, Y. Chang, Y. Guan, X. Li and G. Zeng, J. Hazard. Mater., 2009, 169, 933–940 CrossRef CAS PubMed.
- T.-x. Liu, X.-z. Li and F.-b. Li, Chem. Eng. J., 2010, 157, 475–482 CrossRef CAS PubMed.
- M. Y. Masoomi and A. Morsali, Coord. Chem. Rev., 2012, 256, 2921–2943 CrossRef CAS PubMed.
- A. Morsali and M. Y. Masoomi, Coord. Chem. Rev., 2009, 253, 1882–1905 CrossRef CAS PubMed.
- F. Li, C. Liu, Z. Ma and L. Zhao, Opt. Mater., 2012, 34, 1062–1066 CrossRef CAS PubMed.
- M. G. Nair, M. Nirmala, K. Rekha and A. Anukaliani, Mater. Lett., 2011, 65, 1797–1800 CrossRef CAS PubMed.
- S. Liu, X. Wang, W. Zhao, K. Wang, H. Sang and Z. He, J. Alloys Compd., 2013, 568, 84–91 CrossRef CAS PubMed.
- H. Kou, L. Jia and C. Wang, Carbon, 2012, 50, 3522–3529 CrossRef CAS PubMed.
- R. Wahab, I. H. Hwang, Y.-S. Kim, J. Musarrat, M. A. Siddiqui, H.-K. Seo, S. K. Tripathy and H.-S. Shin, Chem. Eng. J., 2011, 175, 450–457 CrossRef CAS PubMed.
- Y. Wang, X. Li, G. Lu, G. Chen and Y. Chen, Mater. Lett., 2008, 62, 2359–2362 CrossRef CAS PubMed.
- R. C. Pawar, J. S. Shaikh, A. A. Babar, P. M. Dhere and P. S. Patil, Sol. Energy, 2011, 85, 1119–1127 CrossRef CAS PubMed.
- T. T. Vu, L. del Río, T. Valdés-Solís and G. Marbán, J. Hazard. Mater., 2013, 246–247, 126–134 CrossRef CAS PubMed.
- P. Gao, K. Ng and D. D. Sun, J. Hazard. Mater., 2013, 262, 826–835 CrossRef CAS PubMed.
- A. Omidi, A. Habibi-Yangjeh and M. Pirhashemi, Appl. Surf. Sci., 2013, 276, 468–475 CrossRef CAS PubMed.
- G. A. S. Josephine and A. Sivasamy, Appl. Catal., B, 2014, 150–151, 288–297 CrossRef CAS PubMed.
- A. Yousef, N. A. M. Barakat, T. Amna, A. R. Unnithan, S. S. Al-Deyab and H. Yong Kim, J. Lumin., 2012, 132, 1668–1677 CrossRef CAS PubMed.
- H. Kou, X. Zhang, Y. Jiang, J. Li, S. Yu, Z. Zheng and C. Wang, Electrochim. Acta, 2011, 56, 5575–5581 CrossRef CAS PubMed.
- C.-H. Wu, J.-S. Ma, S.-H. Lin and C.-H. Lu, Sol. Energy Mater. Sol. Cells, 2013, 112, 47–51 CrossRef CAS PubMed.
- S. Mehdaoui, N. Benslim, O. Aissaoui, M. Benabdeslem, L. Bechiri, A. Otmani, X. Portier and G. Nouet, Mater. Charact., 2009, 60, 451–455 CrossRef CAS PubMed.
- F. Shen, W. Que, Y. He, Y. Yuan, X. Yin and G. Wang, ACS Appl. Mater. Interfaces, 2012, 4, 4087–4092 CAS.
- Standard Methods for the Examination of Water and Wastewater, 5220 B CHEMICAL OXYGEN DEMAND (COD) Open Reflux Method, 20th Ed., American Public Health Association, 1997 Search PubMed.
- J. G. Lu, P. Chang and Z. Fan, Mater. Sci. Eng., R, 2006, 52, 49–91 CrossRef PubMed.
- Y. Wang, Z. Hou, H. Guo, L. Shen, G. Wang, F. Cui and Q. Zhang, Mater. Lett., 2013, 91, 107–110 CrossRef CAS PubMed.
- S. J. Yang, J. H. Im, T. Kim, K. Lee and C. R. Park, J. Hazard. Mater., 2011, 186, 376–382 CrossRef CAS PubMed.
- C. Chandrinou, N. Boukos, C. Stogios and A. Travlos, Microelectron. J., 2009, 40, 296–298 CrossRef CAS PubMed.
- S. C. Navale and I. S. Mulla, Mater. Sci. Eng., C, 2009, 29, 1317–1320 CrossRef CAS PubMed.
- M. Bagheri, A. A. Khodadadi, A. R. Mahjoub and Y. Mortazavi, Sens. Actuators, B, 2013, 188, 45–52 CrossRef CAS PubMed.
- B. Li, Y. Xie, J. Huang and Y. Qian, Adv. Mater., 1999, 11, 1456–1459 CrossRef CAS.
- L. Zhang, J. Liang, S. Peng, Y. Shi and J. Chen, Mater. Chem. Phys., 2007, 106, 296–300 CrossRef CAS PubMed.
- K. Choi, T. Kang and S.-G. Oh, Mater. Lett., 2012, 75, 240–243 CrossRef CAS PubMed.
- M. Bagheri, N. F. Hamedani, A. R. Mahjoub, A. A. Khodadadi and Y. Mortazavi, Sens. Actuators, B, 2014, 191, 283–290 CrossRef CAS PubMed.
- F. Mohd Omar, H. Abdul Aziz and S. Stoll, Sci. Total Environ., 2014, 468–469, 195–201 CrossRef CAS PubMed.
- G. Cao, Nanostructures and Nanomaterials Synthesis, Properties and Applications, Imperial College Press, London, 2004 Search PubMed.
- R. Rahimi, H. Kerdari, M. Rabbani and M. Shafiee, Desalination, 2011, 280, 412–418 CrossRef CAS PubMed.
- Q. Zhang, Y. Li, E. A. Ackerman, M. Gajdardziska-Josifovska and H. Li, Appl. Catal., A, 2011, 400, 195–202 CrossRef CAS PubMed.
- Y. Hou, L. Wu, X. Wang, Z. Ding, Z. Li and X. Fu, J. Catal., 2007, 250, 12–18 CrossRef CAS PubMed.
- H. Li, S. Yin, Y. Wang and T. Sato, Appl. Catal., B, 2013, 132–133, 487–492 CrossRef CAS PubMed.
- B. Palanisamy, C. M. Babu, B. Sundaravel, S. Anandan and V. Murugesan, J. Hazard. Mater., 2013, 252–253, 233–242 CrossRef CAS PubMed.
- Y. Zhang, M. K. Ram, E. K. Stefanakos and D. Y. Goswami, J. Nanomater., 2012, 2012, 22 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra01735f |
| This journal is © The Royal Society of Chemistry 2014 |