A convenient synthesis of pyrimidinone and pyrimidine containing bisheteroarenes and analogs
Hardesh K.
Maurya
and
Atul
Gupta
*
Medicinal Chemistry Department, Central Institute of Medicinal and aromatic Plants, PO. CIMAP, Kukrail Road, Lucknow-226015, India. E-mail: atisky2001@yahoo.co.in; hardesh11@yahoo.co.in; Tel: +91-5222718556
First published on 7th May 2014
Abstract
The synthesis of pyrimidinone containing bisheteroarenes (3) and related analogs (9 and 10) by the reaction of active methylenes or substituted methyl acrylate with nitrogen containing precursors viz. amidines, or thiourea in water as well as other organic solvents was studied. Synthesized compounds have further been explored for the synthesis of diversified pyrimidines 4, 6–8, 11, 12 and 14 through a sequential approach.
Introduction
Diversified naturally occurring pyrimidinones, pyrimidines and their structural implication on biological systems1 has drawn attention of organic chemists towards further exploration of their chemistry. Among other nitrogen heterocycles, pyrimidinone and pyrimidines are the main constituents of many natural products such as farinamycin (i, isolated from Streptomyces griseus),1a terremide-B (ii),1b 4-thiouridine (iii, isolated from Escherichia coli),1c lathyrine (iv, extracted from L. tingitanus), thiamine (v, vitamine B1, isolated from ricebran), varioline B (vi, isolated from a marine organism kirkpatrickia varialosa); Fig. 1.1dFurther, these molecules form core structure of DNA and RNA (uracil, thymine, cytosine) and are building blocks for many biologically active compounds with established medicinal values.2 Literature search indicated that compounds having pyrimidinone and pyrimidine nucleus possess broad range of biological activities.1–7 Pyrimidinone is a basic nucleus of various clinically useful drugs such as allopurinol (i, free radical scavenger, antimetabolite enzyme inhibitor drug),3 raltegravir (ii, antiretroviral HIV drug),4 thiadiazolopyrimidinones (iii, anti-osteoporosis),5 temelastine (iv, antihistaminic drug),5 lamivudine (v, anti-AIDS drug) (Fig. 2).6
Similarly, pyrimidine is also an integral part in various important drugs such as mezilamine (vi, antipsychotic drug),6 pyrimethamine (vii, antimalarials drugs),1 sulfadimidine (viii, sulfonamide antibacterial drug),6 epirazole (ix, anti-ulcer drug)6 and trimethoprim (x, bacteriostatic antibiotic drugs),1,7 as well as other well known drugs (Fig. 2).6,7
The importance of these molecules in drug development1–7 inspired us to synthesize bisheteroarenes which possess a combo structure of pyrimidine and azaheterocycles in anticipation of their utility in synthesis of biologically active compounds.
Results and discussion
Chemistry
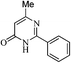
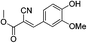
Water is a green solvent and received considerable attention in organic chemistry due to profound economic, environmental, safety and societal advantages over conventional reactions in organic solvents.8 Organic reactions performed in water have shown unique properties towards improved selectivity. Various reactions have been reported in aqueous medium with substantial rate acceleration, even water-insoluble substrates have been used in a suspension to yield products.8 Therefore water is a cheapest, non hazardous and universal solvent which needs continuous efforts to study various synthetic reactions in aqueous medium.Use of sodium in absolute ethanol or methanol under extremely dry conditions is often required for synthesis of pyrimidinone (3).9 In our efforts to synthesize pyrimidinone under very simple reaction conditions, we studied synthesis of substituted pyrimidinones in aqueous medium. We first explored preparation of 6-methyl-2-phenylpyrimidin-4(3H)-one (3a) from benzimidamide (2a) with ethyl 3-oxobutanoate (1a). This reaction was previously reported in refluxing ethanol with 86–98% yields, as depicted in Table 1.9a–d The typical reaction conditions involved refluxing of 2a with ethyl 3-oxobutanoate (1a) in presence of strong base such as sodium or EtONa in dry ethanol (Table 1).9a–d In contrast, when a mixture of 1a and 2a was stirred in presence of KOH in water, it was completed within 48 h leading to the formation of 3a in 97% yield as shown in Table 1. Therefore, it was concluded that water could be a medium of choice over sodium in dry ethanol. This method offered easy purification of products as pure product precipitated on reaction completion which could be isolated by simple filtration.
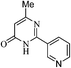
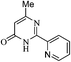
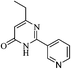
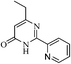
On the basis of above results, we performed various reactions of alkyl 3-oxobutanoate (1a and 1b) with benzimidamide (2a) isonicotinimidamide (2b), nicotinimidamide (2c), picolinimidamide (2d) and 1H-pyrazole-1-carboximidamide (2e) in aqueous medium which proceeded by the condensation and subsequently cyclization as shown in Scheme 1, Table 2.
 | ||
| Scheme 1 Synthesis of pyrimidinone containing bisheteroarenes (3) in water (Table 2). | ||
To explore the immediate utility of synthesized pyrimidinone (3), we explored reaction of 6-methyl-2-phenylpyrimidin-4(3H)-one (3a) in POCl3 at 140 °C for the formation of chlorinated pyrimidine (4) which was susceptible for substitution of chlorine by nucleophile such as ethyl thioglyconate etc.
Further, reaction of chlorinated pyrimidine (4) with ethyl thioglyconate and K2CO3 in refluxing ethanol yielded ethyl 2-(6-methyl-2-phenylpyrimidin-4-ylthio)acetate (5) and hydrolyzed product (6); Scheme 2 (Table 3). However, above reaction in NaH/THF produced regioselectively pyrimidine (5). In order to prepare hydrazone containing derivative as present in anti-tubercular drug isoniazide, pyrimidine (5) was converted into 2-(6-methyl-2-phenylpyrimidin-4-ylthio)acetohydrazide (7) in presence of hydrazine in ethanol at reflux temperature in anticipation of its anti-tubercular activity; Scheme 2.
 | ||
| Scheme 2 Pyrimidinone (3a) applied as precursor for the synthesis of pyrimidines (4, 5–7) (Table 3). | ||
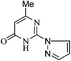
The aza Michael type reaction of benzimidamide (2a) with methyl 2-cyano-3,3-bis(methylthio)acrylate (8) in aqueous medium formed pyrimidinone (9a) in 10% yield. However, when same reaction was performed in DMF or ethanol, the yield of product was 42–85% (Table 4). Thus, other pyrimidinone (9b and 9c) were prepared in DMF or ethanol as shown in Scheme 3 (Table 4).
 | ||
| Scheme 3 Synthesis of pyrimidinone containing bisheteroarenes (9), (Table 4). | ||
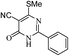
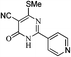
Toward the study for diversification of synthesized pyrimidinone (9), we further explored the reaction of pyrimidin-4(3H)-one (9) and hydrazine in refluxing ethanol which yielded 1H-pyrazolo[3,4-d]pyrimidin-4(5H)-one (10) in 56% yield, while the reaction of pyrimidin-4(3H)-one (9) in POCl3 at 140 °C yielded chlorinated pyrimidine (11). Compound 11 on reaction with ethyl thioglyconate (5) in presence of Et3N in refluxing ethanol produced mono substituted product (12) instead of anticipated cyclic product (13). However, when the reaction of 11 and 5 was attempted in NaH/THF, compound 14 was obtained in 53% yield instead of target compound 13 (Scheme 4, Table 5).
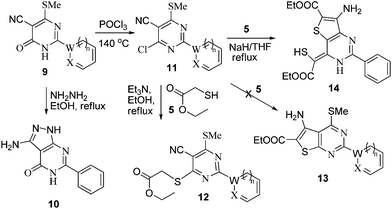 | ||
| Scheme 4 Pyrimidinone (9) applied as precursor for the synthesis of pyrimidine containing bisheteroarenes (10–12) (Table 5). | ||
After the synthetic study of pyrimidinone (3 and 9) by the reaction of amidine (2) with ethyl 3-oxobutanoate (1) and 4 respectively, we intended to synthesize new pyrimidinone analogs via multi component reaction. Initially, we attempted reaction of thiourea (15), methyl 2-cyanoacetate (16) and benzaldehyde (17) in ethanol at reflux temperature which produced 4-oxo-2-thioxo-6-p-tolyl-1,2,3,4-tetrahydropyrimidine-5-carbonitrile (18) in good yield. Further, reaction of pyrimidinone (18) with ethyl 2-bromoacetate (19) in presence of K2CO3 in DMF yielded mono and disubstiuted pyrimidine derivative 20 and 21. In anticipation to synthesize pyrimidinone (18) in aqueous medium, we further explored the reaction of thiourea (15), methyl 2-cyanoacetate (16), benzaldehyde (17) in presence of K2CO3 in water which gave a precipitate within a minute. For complete consumption of residual starting materials, the reaction mixture was further stirred for 5–15 minutes. The obtained precipitate was filtered as pure product which did not require any further purification or crystallization. The product was identified as (E)-methyl 2-cyano-3-(4-aryl) acrylate (22) instead of anticipated product pyrimidinone (18), Scheme 5 (Table 6).
 | ||
| Scheme 5 Synthesis of pyrimidinone (18) and (E)-methyl 2-cyano-3-(4-aryl)acrylate (22) applied as precursor for the synthesis of pyrimidine (20 and 21) (Table 6). | ||
Conclusion
In conclusion, a convenient synthesis of pyrimidinone containing bisheteroarenes and analogs by the reaction of nitrogen containing precursors viz. amidines, thiourea with active methylenes or acrylate in water as well as other organic solvents had been developed. These compounds were further explored the synthesis of diversified pyrimidines through sequential approach. The optimized reaction condition will be further explored for synthesis of various biological active compounds.Experimental section
General
The reagents and solvents used in this study were of analytical grade and used without further purification. All the reactions were monitored on Merck aluminum thin layer chromatography (TLC, UV254nm) plates. Column chromatography was carried out on silica gel (60–120 mesh). The melting points were determined on Buchi melting point M560 apparatus in open capillaries and are uncorrected. Commercial reagents were used without purification.1H (300 MHz) and 13C NMR (75 MHz) spectra were recorded on a Bruker WM-300 using CDCl3 and DMSO-d6 as the solvent. Chemical shift are reported in parts per million shift (δ-value) based on the middle peak of the solvent (CDCl3/DMSO-d6) as the internal standard. Signal patterns are indicated as s-singlet; bs-broad singlet; d-doublet; dd-double doublet; t-triplet; m-multiplet; brm-broad multiplet. Coupling constants (J) are given in Hertz. Infrared (IR) spectra were recorded on a Perkin-Elmer AX-1 spectrophotometer in KBr disc and reported in wave number (cm−1). ESI mass spectra were recorded on Shimadzu LC-MS after dissolving the compounds in acetonitrile and methanol.General procedure for the synthesis of 6-alkyl-2-heteroaryl/phenylpyrimidin-4(3H)-one (3)
A mixture of amidine (2, 4 mmol) and potassium hydroxide (4.1 mmol) in water (10 mL) was stirred for 5 minutes at room temperature and subsequently ethyl 3-oxoalkanoate (1, 4.1 mmol) were added to this solution. Th content was stirred for 24–96 h (Table 1) at room temperature. After completion of the reaction, the aqueous phase was neutralized with N/10 HCl. The precipitate obtained was filtered (or extract with excess ethyl acetate, in case precipitate did not formed) and recrystallized with methanol as pure bisheteroarene (3).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 3449 (NH); UV(CH3OH, λmax/nm−1 (ε/dm−3 mol−1 cm−1): 238 nm (1300), 285 nm (700); 1H NMR (DMSO-d6, 300 MHz, δ ppm): 2.24 (s, 3H, CH3), 6.18 (s, 1H, CH), 7.45–7.57 (m, 3H, Ar-H), 8.04–8.07 (m, 2H, Ar-H), 8.25 (s, 1H, NH); 13C NMR (DMSO-d6, 75 MHz, δ ppm): 24.2 (CH3), 110.7 (CH), 128.5 (2 × Ar-H), 129.4 (2 × Ar-H), 132.3 (Ar-H), 133.5, 157.8, 164.7, 165.2 (C
O), 3449 (NH); UV(CH3OH, λmax/nm−1 (ε/dm−3 mol−1 cm−1): 238 nm (1300), 285 nm (700); 1H NMR (DMSO-d6, 300 MHz, δ ppm): 2.24 (s, 3H, CH3), 6.18 (s, 1H, CH), 7.45–7.57 (m, 3H, Ar-H), 8.04–8.07 (m, 2H, Ar-H), 8.25 (s, 1H, NH); 13C NMR (DMSO-d6, 75 MHz, δ ppm): 24.2 (CH3), 110.7 (CH), 128.5 (2 × Ar-H), 129.4 (2 × Ar-H), 132.3 (Ar-H), 133.5, 157.8, 164.7, 165.2 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); MS (C11H10N2O): 185 [M − H]+, 187 [M + H]+, 209 [ M + Na]+.
O); MS (C11H10N2O): 185 [M − H]+, 187 [M + H]+, 209 [ M + Na]+.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 3432 (NH); 1H NMR (DMSO-d6, 300 MHz, δ ppm): 2.28 (s, 3H, CH3), 6.34 (s, 1H, CH), 7.99 (d, J = 4.8 Hz, 2H, Ar-H), 8.69 (d, J = 4.8 Hz, 2H, Ar-H); 13C NMR (DMSO-d6, 75 MHz, δ ppm): 24.1 (CH3), 110.9 (CH), 122.4 (2 × Ar-H), 141.8, 151.0 (2 × Ar-H), 156.4, 162.5, 166.1 (C
O), 3432 (NH); 1H NMR (DMSO-d6, 300 MHz, δ ppm): 2.28 (s, 3H, CH3), 6.34 (s, 1H, CH), 7.99 (d, J = 4.8 Hz, 2H, Ar-H), 8.69 (d, J = 4.8 Hz, 2H, Ar-H); 13C NMR (DMSO-d6, 75 MHz, δ ppm): 24.1 (CH3), 110.9 (CH), 122.4 (2 × Ar-H), 141.8, 151.0 (2 × Ar-H), 156.4, 162.5, 166.1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); MS (C10H9N3O): 188.2 [M + H]+.
O); MS (C10H9N3O): 188.2 [M + H]+.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 3434 (NH); 1H NMR (DMSO-d6, 300 MHz, δ ppm): 2.15 (s, 3H, CH3), 6.14 (s, 1H, CH), 7.37–7.41 (m, 1H, Ar-H), 8.13 (s, 1H, NH), 8.26–8.29 (m, 1H, Ar-H), 8.55–8.57 (m, 1H, Ar-H), 9.08 (s, 1H, Ar-H); 13C NMR (DMSO-d6, 75 MHz, δ ppm): 24.20 (CH3), 110.4 (CH), 124.4 (Ar-H), 130.2, 136.2 (Ar-H), 149.5 (Ar-H), 152.5 (Ar-H), 153.0, 157.2, 165.7 (C
O), 3434 (NH); 1H NMR (DMSO-d6, 300 MHz, δ ppm): 2.15 (s, 3H, CH3), 6.14 (s, 1H, CH), 7.37–7.41 (m, 1H, Ar-H), 8.13 (s, 1H, NH), 8.26–8.29 (m, 1H, Ar-H), 8.55–8.57 (m, 1H, Ar-H), 9.08 (s, 1H, Ar-H); 13C NMR (DMSO-d6, 75 MHz, δ ppm): 24.20 (CH3), 110.4 (CH), 124.4 (Ar-H), 130.2, 136.2 (Ar-H), 149.5 (Ar-H), 152.5 (Ar-H), 153.0, 157.2, 165.7 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); MS (C10H9N3O): 188 [M + H]+.
O); MS (C10H9N3O): 188 [M + H]+.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 3435 (NH); 1H NMR (DMSO-d6, 300 MHz, δ ppm): 2.13 (s, 3H, CH3), 6.09 (s, 1H, CH), 7.46–7.50 (m, 1H, Ar-H), 7.84–7.90 (m, 1H, Ar-H), 8.12–8.15 (m, 2H, Ar-H & NH), 8.57 (d, J = 3.9 Hz, 1H, Ar-H); 13C NMR (DMSO-d6, 75 MHz, δ ppm): 23.7 (CH3), 113.0 (CH), 123.2 (Ar-H), 127.5, 138.8 (Ar-H), 149.2 (Ar-H), 150.0 (Ar-H), 154.9, 163.3 (C
O), 3435 (NH); 1H NMR (DMSO-d6, 300 MHz, δ ppm): 2.13 (s, 3H, CH3), 6.09 (s, 1H, CH), 7.46–7.50 (m, 1H, Ar-H), 7.84–7.90 (m, 1H, Ar-H), 8.12–8.15 (m, 2H, Ar-H & NH), 8.57 (d, J = 3.9 Hz, 1H, Ar-H); 13C NMR (DMSO-d6, 75 MHz, δ ppm): 23.7 (CH3), 113.0 (CH), 123.2 (Ar-H), 127.5, 138.8 (Ar-H), 149.2 (Ar-H), 150.0 (Ar-H), 154.9, 163.3 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 164.0; MS (C10H9N3O): 188 [M + H]+.
O), 164.0; MS (C10H9N3O): 188 [M + H]+.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 3434 (NH); 1H NMR (CDCl3, 300 MHz, δ ppm): 1.32 (t, J = 7.5 Hz, 3H, CH3), 2.69 (q, J = 7.5 Hz, 2H, CH2), 6.42 (s, 1H, CH), 7.27 (s, 1H, NH), 8.17 (d, J = 4.8 Hz, 2H, Ar-H), 8.82 (d, J = 4.8 Hz, 2H, Ar-H); 13C NMR (CDCl3, 75 MHz, δ ppm): 12.4 (CH3), 31.1 (CH2), 111.2 (CH), 121.8 (2 × Ar-H), 139.9, 151.1 (2 × Ar-H), 154.6, 166.1 (C
O), 3434 (NH); 1H NMR (CDCl3, 300 MHz, δ ppm): 1.32 (t, J = 7.5 Hz, 3H, CH3), 2.69 (q, J = 7.5 Hz, 2H, CH2), 6.42 (s, 1H, CH), 7.27 (s, 1H, NH), 8.17 (d, J = 4.8 Hz, 2H, Ar-H), 8.82 (d, J = 4.8 Hz, 2H, Ar-H); 13C NMR (CDCl3, 75 MHz, δ ppm): 12.4 (CH3), 31.1 (CH2), 111.2 (CH), 121.8 (2 × Ar-H), 139.9, 151.1 (2 × Ar-H), 154.6, 166.1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 171.8; MS (C11H11N3O): 202.2 [M + H]+.
O), 171.8; MS (C11H11N3O): 202.2 [M + H]+.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 3430 (NH); 1H NMR (DMSO-d6, 300 MHz, δ ppm): 1.16 (t, J = 7.5 Hz, 3H, CH3), 2.51 (q, J = 7.5 Hz, 2H, CH2), 6.20 (s, 1H, CH), 7.47–7.51 (m, 1H, Ar-H), 8.40–8.43 (m, 1H, Ar-H), 8.65–8.67 (m, 1H, Ar-H), 8.82 (s, 1H), 9.18 (s, 1H, Ar-H); 13C NMR (DMSO-d6, 75 MHz, δ ppm): 13.1 (CH3), 30.6 (CH2), 108.8 (CH), 124.3 (Ar-H), 131.1, 136.1 (Ar-H), 149.6 (Ar-H), 152.2 (Ar-H), 158.0, 167.3 (C
O), 3430 (NH); 1H NMR (DMSO-d6, 300 MHz, δ ppm): 1.16 (t, J = 7.5 Hz, 3H, CH3), 2.51 (q, J = 7.5 Hz, 2H, CH2), 6.20 (s, 1H, CH), 7.47–7.51 (m, 1H, Ar-H), 8.40–8.43 (m, 1H, Ar-H), 8.65–8.67 (m, 1H, Ar-H), 8.82 (s, 1H), 9.18 (s, 1H, Ar-H); 13C NMR (DMSO-d6, 75 MHz, δ ppm): 13.1 (CH3), 30.6 (CH2), 108.8 (CH), 124.3 (Ar-H), 131.1, 136.1 (Ar-H), 149.6 (Ar-H), 152.2 (Ar-H), 158.0, 167.3 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 170.2; MS (C11H11N3O): 202.2 [M + H]+.
O), 170.2; MS (C11H11N3O): 202.2 [M + H]+.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 3448 (NH); 1H NMR (DMSO-d6, 300 MHz, δ ppm): 1.26 (t, J = 7.8 Hz, 3H, CH3), 2.60 (q, J = 7.8 Hz, 2H, CH2), 6.28 (s, 1H, CH), 7.43 (t, J = 7.2 Hz, 1H, Ar-H), 7.85 (t, J = 7.5 Hz, 1H, Ar-H), 8.41 (d, J = 7.8 Hz, 1H, Ar-H), 8.60 (s, 1H), 10.95 (bs, 1H, NH); 13C NMR (DMSO-d6, 75 MHz, δ ppm): 12.5 (CH3), 31.0 (CH2), 112.2 (CH), 122.5 (Ar-H), 126.8 (Ar-H), 137.9 (Ar-H), 148.3, 149.2, (Ar-H), 153.3, 162.5 (C
O), 3448 (NH); 1H NMR (DMSO-d6, 300 MHz, δ ppm): 1.26 (t, J = 7.8 Hz, 3H, CH3), 2.60 (q, J = 7.8 Hz, 2H, CH2), 6.28 (s, 1H, CH), 7.43 (t, J = 7.2 Hz, 1H, Ar-H), 7.85 (t, J = 7.5 Hz, 1H, Ar-H), 8.41 (d, J = 7.8 Hz, 1H, Ar-H), 8.60 (s, 1H), 10.95 (bs, 1H, NH); 13C NMR (DMSO-d6, 75 MHz, δ ppm): 12.5 (CH3), 31.0 (CH2), 112.2 (CH), 122.5 (Ar-H), 126.8 (Ar-H), 137.9 (Ar-H), 148.3, 149.2, (Ar-H), 153.3, 162.5 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 170.5; MS (C11H11N3O): 202.2 [M + H]+.
O), 170.5; MS (C11H11N3O): 202.2 [M + H]+.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 3480 (NH); UV(CH3OH), λmax/nm−1 (ε/dm−3 mol−1 cm−1): 258 nm (900), 287 nm (475), 310 (300); 1H NMR (DMSO-d6, 300 MHz, δ ppm): 2.29 (s, 3H, CH3), 6.30 (s, 1H, CH), 6.54 (t, J = 1.5 Hz, 1H, pyrazol-H), 7.81 (s, 1H, pyrazol-H), 8.50 (s, 1H, pyrazol-H); 13C NMR (DMSO-d6, 75 MHz, δ ppm): 23.8 (CH3), 106.4 (CH), 109.4 (pyrazol-H), 110.4, 129.9 (pyrazol-H), 143.9 (pyrazol-H), 152.7, 168.9 (C
O), 3480 (NH); UV(CH3OH), λmax/nm−1 (ε/dm−3 mol−1 cm−1): 258 nm (900), 287 nm (475), 310 (300); 1H NMR (DMSO-d6, 300 MHz, δ ppm): 2.29 (s, 3H, CH3), 6.30 (s, 1H, CH), 6.54 (t, J = 1.5 Hz, 1H, pyrazol-H), 7.81 (s, 1H, pyrazol-H), 8.50 (s, 1H, pyrazol-H); 13C NMR (DMSO-d6, 75 MHz, δ ppm): 23.8 (CH3), 106.4 (CH), 109.4 (pyrazol-H), 110.4, 129.9 (pyrazol-H), 143.9 (pyrazol-H), 152.7, 168.9 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); MS (C8H8N4O): 175 [M − H]+, 199 [M + Na]+.
O); MS (C8H8N4O): 175 [M − H]+, 199 [M + Na]+.
General procedure for the synthesis of 4-chloro-6-methyl-2-phenylpyrimidine (4)
Bisheteroarene (3a, 10 mmol) in POCl3 (10 mL) was refluxed at 140 °C for 15 h. After completion, the reaction mixture was cooled and poured onto crushed ice with vigorous stirring and the precipitate obtained was filtered, washed with water and dried. Residue was recrystallized with hexane. white crystalline solid; Rf: 0.50 (5% ethyl acetate–hexane); UV(CH3OH), λmax/nm−1 (ε/dm−3 mol−1 cm−1): 258 nm (2000); 1H NMR (CDCl3, 300 MHz, δ ppm): 2.56 (s, 3H, CH3), 7.08 (s, 1H, CH), 7.48 (m, 3H, Ar-H), 8.44 (m, 2H, Ar-H); 13C NMR (CDCl3, 75 MHz, δ ppm): 24.4 (CH3), 118.7 (CH), 128.9 (4 × Ar-H), 131.6 (Ar-H), 136.8, 161.7, 165.4, 169.3; MS (C11H9ClN2): 242 [M + K − H]+.Synthesis of ethyl 2-(6-methyl-2-phenylpyrimidin-4-ylthio)-acetate (5) & 2-(6-methyl-2-phenylpyrimidin-4-ylthio)acetic acid (6)
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); 1H NMR (CDCl3, 300 MHz, δ ppm): 1.24 (t, J = 7.2 Hz, 3H, CH3), 2.44 (s, 3H, CH3), 4.01 (s, 2H, SCH2), 4.19 (q, J = 7.2 Hz, 2H, OCH2) 6.94 (s, 1H, CH), 7.44 (t, J = 0.5 Hz, 3H, Ar-H), 8.42 (d, J = 0.5 Hz, 2H, Ar-H); 13C NMR (CDCl3, 75 MHz, δ ppm): 14.5 (CH3), 24.3 (CH3), 32.2 (SCH2), 62.1 (OCH2), 116.1 (CH), 128.7 (Ar-H), 128.9 (Ar-H), 129.0 (Ar-H), 129.6 (Ar-H), 131.1 (Ar-H), 137.8, 163.7, 165.5, 167.7 (C–S), 169.5 (C
O); 1H NMR (CDCl3, 300 MHz, δ ppm): 1.24 (t, J = 7.2 Hz, 3H, CH3), 2.44 (s, 3H, CH3), 4.01 (s, 2H, SCH2), 4.19 (q, J = 7.2 Hz, 2H, OCH2) 6.94 (s, 1H, CH), 7.44 (t, J = 0.5 Hz, 3H, Ar-H), 8.42 (d, J = 0.5 Hz, 2H, Ar-H); 13C NMR (CDCl3, 75 MHz, δ ppm): 14.5 (CH3), 24.3 (CH3), 32.2 (SCH2), 62.1 (OCH2), 116.1 (CH), 128.7 (Ar-H), 128.9 (Ar-H), 129.0 (Ar-H), 129.6 (Ar-H), 131.1 (Ar-H), 137.8, 163.7, 165.5, 167.7 (C–S), 169.5 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); MS (C15H16N2O2S): 289.2 [M + H]+.
O); MS (C15H16N2O2S): 289.2 [M + H]+.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 3447 (OH); 1H NMR (CDCl3, 300 MHz, δ ppm): 2.30 (s, 3H, CH3), 3.95 (s, 2H, SCH2), 6.59 (s, 1H, CH), 7.41 (m, 3H, Ar-H), 8.31 (m, 2H, Ar-H), 9.31 (bs, 1H, OH); 13C NMR (CDCl3, 75 MHz, δ ppm): 24.3 (CH3), 32.6 (SCH2), 116.3 (CH), 128.7 (2 × Ar-H), 128.9 (2 × Ar-H), 131.4 (Ar-H), 137.1, 164.1, 166.2, 167.8 (C–S), 173.2 (C
O), 3447 (OH); 1H NMR (CDCl3, 300 MHz, δ ppm): 2.30 (s, 3H, CH3), 3.95 (s, 2H, SCH2), 6.59 (s, 1H, CH), 7.41 (m, 3H, Ar-H), 8.31 (m, 2H, Ar-H), 9.31 (bs, 1H, OH); 13C NMR (CDCl3, 75 MHz, δ ppm): 24.3 (CH3), 32.6 (SCH2), 116.3 (CH), 128.7 (2 × Ar-H), 128.9 (2 × Ar-H), 131.4 (Ar-H), 137.1, 164.1, 166.2, 167.8 (C–S), 173.2 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); MS (C13H12N2O2S): 259 [M − H]+, 259 [M − 2Na]+.
O); MS (C13H12N2O2S): 259 [M − H]+, 259 [M − 2Na]+.
Synthesis of 2-(6-methyl-2-phenylpyrimidin-4-ylthio)aceto-hydrazide (7)
Pyrimidine (5, 1.0 mmol) and hydrazine hydrate (1 mL) in ethanol (40 mL) were refluxed with vigorous stirring for 10 h. After the completion of reaction, reaction mixture was left for overnight. Precipitate was formed which was filtered and recrystallized with dichloromethane. Rf: 0.13 (4% chloroform); IR (KBr, νmax/cm−1): 1661 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 3288 (NH); 1H NMR (DMSO-d6, 300 MHz, δ ppm): 2.41 (s, 3H, CH3), 4.02 (s, 2H, SCH2), 7.22 (s, 1H, CH), 7.48–7.50 (m, 3H, Ar-H), 8.31-8.34 (m, 2H, Ar-H), 9.46 (bs, 1H, NH); 13C NMR (DMSO-d6, 75 MHz, δ ppm): 24.2 (CH3), 31.7 (SCH2), 116.4 (CH), 128.7 (2 × Ar-H), 129.5 (2 × Ar-H), 131.8 (Ar-H), 137.5, 163.2, 166.4, 168.1 (C–S), 169.2 (C
O), 3288 (NH); 1H NMR (DMSO-d6, 300 MHz, δ ppm): 2.41 (s, 3H, CH3), 4.02 (s, 2H, SCH2), 7.22 (s, 1H, CH), 7.48–7.50 (m, 3H, Ar-H), 8.31-8.34 (m, 2H, Ar-H), 9.46 (bs, 1H, NH); 13C NMR (DMSO-d6, 75 MHz, δ ppm): 24.2 (CH3), 31.7 (SCH2), 116.4 (CH), 128.7 (2 × Ar-H), 129.5 (2 × Ar-H), 131.8 (Ar-H), 137.5, 163.2, 166.4, 168.1 (C–S), 169.2 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); MS (C13H14N4OS): 275.2 [M + H]+, 325.2 [M + K]+.
O); MS (C13H14N4OS): 275.2 [M + H]+, 325.2 [M + K]+.
Synthesis of 1,6-dihydro-4-(methylthio)-6-oxo-2-heteroaryl/phenylpyrimidine-5-carbonitrile (9)
A mixture of amidine (2, 2.0 mmol) and methyl 2-cyano-3,3-dimethylthioacrylate (8, 2.0 mmol) in DMF (10 mL) or EtOH (20 mL) in the presence of powdered KOH or NaOH (2.1 mmol) or Et3N (3 mmol) was stirred at room temperature or reflux as shown in Table 3. The reaction was monitored using TLC. After consumption of 8, the residue was poured onto crushed ice with vigorous stirring. The aqueous suspension was neutralized with N/10 HCl (if required). The precipitate obtained was filtered, washed with water and dried. Further washing with cold methanol yielded 9 as white solid.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 2223 (CN), 3440 (NH); UV(CH3OH), λmax/nm−1 (ε/dm−3 mol−1 cm−1): 238 nm (1200), 285 nm (700); 1H NMR (DMSO-d6, 300 MHz, δ ppm): 2.49 (s, 3H, SCH3), 7.34 (t, J = 3.0 Hz, 3H, Ar-H), 8.21 (t, J = 3.0 Hz, 2H, Ar-H); 13C NMR (DMSO-d6, 75 MHz, δ ppm): 12.6 (SCH3), 89.8, 119.3 (CN), 128.9 (2 × Ar-H), 129.0 (Ar-H), 131.2 (2 × Ar-H), 139.0, 163.8, 171.5, 172.0; MS (C12H9N3OS): 242 [M − H]+, 244 [M + H]+, 282[M + K]+.
O), 2223 (CN), 3440 (NH); UV(CH3OH), λmax/nm−1 (ε/dm−3 mol−1 cm−1): 238 nm (1200), 285 nm (700); 1H NMR (DMSO-d6, 300 MHz, δ ppm): 2.49 (s, 3H, SCH3), 7.34 (t, J = 3.0 Hz, 3H, Ar-H), 8.21 (t, J = 3.0 Hz, 2H, Ar-H); 13C NMR (DMSO-d6, 75 MHz, δ ppm): 12.6 (SCH3), 89.8, 119.3 (CN), 128.9 (2 × Ar-H), 129.0 (Ar-H), 131.2 (2 × Ar-H), 139.0, 163.8, 171.5, 172.0; MS (C12H9N3OS): 242 [M − H]+, 244 [M + H]+, 282[M + K]+.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 2212 (CN), 3441 (NH); 1H NMR (DMSO-d6, 300 MHz, δ ppm): 2.48 (s, 3H, SCH3), 8.09 (t, J = 4.5 Hz, 3H, Ar-H), 8.84 (t, J = 4.5 Hz, 2H, Ar-H); MS (C11H8N4OS): 245.2 [M + H]+.
O), 2212 (CN), 3441 (NH); 1H NMR (DMSO-d6, 300 MHz, δ ppm): 2.48 (s, 3H, SCH3), 8.09 (t, J = 4.5 Hz, 3H, Ar-H), 8.84 (t, J = 4.5 Hz, 2H, Ar-H); MS (C11H8N4OS): 245.2 [M + H]+.
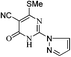
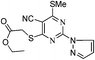
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 2211 (CN), 3480 (NH); UV(CH3OH), λmax/nm−1 (ε/dm−3 mol−1 cm−1): 258 nm (900), 287 nm (400), 310 nm (350); 1H NMR (DMSO-d6, 300 MHz, δ ppm): 2.65 (s, 3H, SCH3), 6.68 (d, J = 1.2 Hz, 1H, pyrazol-H), 7.98 (bs, 1H, pyrazol-H), 8.68 (d, J = 1.2 Hz, 1H, pyrazol-H); 13C NMR (DMSO-d6, 75 MHz, δ ppm): 13.8 (SCH3), 90.3, 111.5 (pyrazol-H), 115.1 (CN), 131.6 (pyrazol-H), 146.1 (pyrazol-H), 150.4, 163.3 (C
O), 2211 (CN), 3480 (NH); UV(CH3OH), λmax/nm−1 (ε/dm−3 mol−1 cm−1): 258 nm (900), 287 nm (400), 310 nm (350); 1H NMR (DMSO-d6, 300 MHz, δ ppm): 2.65 (s, 3H, SCH3), 6.68 (d, J = 1.2 Hz, 1H, pyrazol-H), 7.98 (bs, 1H, pyrazol-H), 8.68 (d, J = 1.2 Hz, 1H, pyrazol-H); 13C NMR (DMSO-d6, 75 MHz, δ ppm): 13.8 (SCH3), 90.3, 111.5 (pyrazol-H), 115.1 (CN), 131.6 (pyrazol-H), 146.1 (pyrazol-H), 150.4, 163.3 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 176.8; MS (C9H7N5OS): 232 [M − H]+, 234 [M + H]+, 256 [M + Na]+.
O), 176.8; MS (C9H7N5OS): 232 [M − H]+, 234 [M + H]+, 256 [M + Na]+.
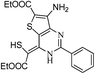
Synthesis of 3-amino-6-phenyl-1H-pyrazolo[3,4-d]pyrimidin-4(5H)-one (10)
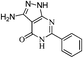
A mixture of pyrimidinone (9, 2 mmol) and hydrazine hydrate (2.5 mmol) in methanol (50 mL) was refluxed (Table 4). After completion of the reaction, methanol was removed under reduced pressure and residue was poured in cold water with stirring. The aqueous phase was neutralized with N/10 HCl (if required). The crude product obtained was filtered, washed with water and finally purified by washing with ether. Cream coloured solid; Rf: 0.57 (EtOAc); IR (KBr, νmax/cm−1): 1645 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 3268, 3419 (NH); UV(CH3OH), λmax/nm−1 (ε/dm−3 mol−1 cm−1): 198 nm (2900), 237 nm (1900); 1H NMR (DMSO-d6, 300 MHz, δ ppm): 5.50 (bs, 2H, NH2), 7.46–7.54 (m, 3H, Ar-H), 7.99 (d, J = 7.2 Hz, 2H, Ar-H), 11.71 (bs, 1H, NH); 13C NMR (DMSO-d6, 75 MHz, δ ppm): 92.0, 128.5 (2 × Ar-H), 129.5 (2 × Ar-H), 132.3 (Ar-H), 133.4, 151.0, 155.1, 155.9, 160.6 (C
O), 3268, 3419 (NH); UV(CH3OH), λmax/nm−1 (ε/dm−3 mol−1 cm−1): 198 nm (2900), 237 nm (1900); 1H NMR (DMSO-d6, 300 MHz, δ ppm): 5.50 (bs, 2H, NH2), 7.46–7.54 (m, 3H, Ar-H), 7.99 (d, J = 7.2 Hz, 2H, Ar-H), 11.71 (bs, 1H, NH); 13C NMR (DMSO-d6, 75 MHz, δ ppm): 92.0, 128.5 (2 × Ar-H), 129.5 (2 × Ar-H), 132.3 (Ar-H), 133.4, 151.0, 155.1, 155.9, 160.6 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); MS (C11H9N5O): 226 [M − H]+, 250[ M + Na]+.
O); MS (C11H9N5O): 226 [M − H]+, 250[ M + Na]+.
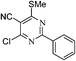
Synthesis of 4-chloro-6-(methylthio)-2-heteroaryl/phenyl pyrimidine-5-carbonitrile (11)
Pyrimidinone (9, 4 mmol) in POCl3 (5 mL) was refluxed at 140 °C (Table 4). After completion, the reaction mixture was cooled and poured onto crushed ice with vigorous stirring. The precipitate obtained was filtered, washed with water and dried and purified by a silica gel column chromatography using solvent ethyl acetate in hexane.Synthesis of ethyl 2-(5-cyano-6-(methylthio)-2-phenyl pyrimidin-4-ylthio)acetate (12a)
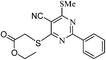
Pyrimidine (11a, 1 mmol), ethyl thioglycolate (1.5 mmol) and Et3N (1.5 mmol) in ethanol (20 mL) were refluxed at 120 °C. After completion of the reaction, ethanol was removed under reduced pressure and residue was poured in cold water with stirring. The aqueous phase was neutralized with N/10 HCl (if required). The crude product obtained was filtered and purified by a silica gel column chromatography using ethyl acetate (0.5–1.0%) in hexane. White solid, Rf: 0.48 (10% EtOAc in hexane); IR (KBr, νmax/cm−1): 2211 (CN), 1736 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); 1H NMR (CDCl3, 300 MHz, δ ppm): 1.26 (t, J = 7.2 Hz, 3H, CH3), 2.74 (s, 3H, SCH3), 4.06 (s, 2H, SCH2), 4.19 (q, J = 7.2 Hz, 2H, OCH2), 7.46–7.57 (m, 3H, Ar-H), 8.43 (t, J = 0.50 Hz, 2H, Ar-H); 13C NMR (CDCl3, 75 MHz, δ ppm): 13.3 (SCH3), 14.5 (CH3), 32.7 (SCH2), 62.5 (OCH2), 100.2, 113.4 (CN), 129.0 (2 × Ar-H), 129.7 (2 × Ar-H), 132.8 (Ar-H), 136.2, 162.5, 168.5 (C
O); 1H NMR (CDCl3, 300 MHz, δ ppm): 1.26 (t, J = 7.2 Hz, 3H, CH3), 2.74 (s, 3H, SCH3), 4.06 (s, 2H, SCH2), 4.19 (q, J = 7.2 Hz, 2H, OCH2), 7.46–7.57 (m, 3H, Ar-H), 8.43 (t, J = 0.50 Hz, 2H, Ar-H); 13C NMR (CDCl3, 75 MHz, δ ppm): 13.3 (SCH3), 14.5 (CH3), 32.7 (SCH2), 62.5 (OCH2), 100.2, 113.4 (CN), 129.0 (2 × Ar-H), 129.7 (2 × Ar-H), 132.8 (Ar-H), 136.2, 162.5, 168.5 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 170.9, 173.5; MS (C16H15N3O2S2): 344[M − H]+, 346 [M + H]+.
O), 170.9, 173.5; MS (C16H15N3O2S2): 344[M − H]+, 346 [M + H]+.
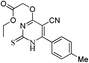
Ethyl 5-amino-4-(methylthio)-2-(1H-pyrazol-1-yl)thieno[2,3-d]pyrimidine-6-carboxylate (12b)
Pyrimidine (11b, 2 mmol), ethyl thioglycolate (2.4 mmol) and Et3N (2.5 mmol) in ethanol (50 mL) were refluxed for 30 h at 120 °C. After completion of the reaction, cool the reaction mixture. Precipitate was formed which was filtered and recrystallized in methanol. White solid, Rf: 0.26 (CHCl3); λmax/nm−1 (ε/dm−3 mol−1 cm−1): 202 (600), 269 (1900); IR (KBr, νmax/cm−1): 2215 (CN), 1737 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); 1H NMR (CDCl3, 300 MHz, δ ppm): 1.27 (t, J = 7.2 Hz, 3H, CH3), 2.77 (s, 3H, SCH3), 4.07 (s, 2H, CH2), 4.19 (q, J = 7.2 Hz, 2H, OCH2), 6.57 (dd, J = 1.5 & 1.2 Hz, 1H, pyrazole-H), 7.87 (d, J = 0.9 Hz, 1H, pyrazole-H), 8.59 (d, J = 0.9 Hz, 1H, pyrazole-H); 13C NMR (CDCl3, 75 MHz, δ ppm): 12.1 (SCH3), 13.1 (CH3), 31.6 (SCH2), 61.1 (OCH2), 97.4, 108.9 (Ar-H), 111.6 (CN), 129.5 (Ar-H), 144.2 (Ar-H), 151.6, 166.9 (C
O); 1H NMR (CDCl3, 300 MHz, δ ppm): 1.27 (t, J = 7.2 Hz, 3H, CH3), 2.77 (s, 3H, SCH3), 4.07 (s, 2H, CH2), 4.19 (q, J = 7.2 Hz, 2H, OCH2), 6.57 (dd, J = 1.5 & 1.2 Hz, 1H, pyrazole-H), 7.87 (d, J = 0.9 Hz, 1H, pyrazole-H), 8.59 (d, J = 0.9 Hz, 1H, pyrazole-H); 13C NMR (CDCl3, 75 MHz, δ ppm): 12.1 (SCH3), 13.1 (CH3), 31.6 (SCH2), 61.1 (OCH2), 97.4, 108.9 (Ar-H), 111.6 (CN), 129.5 (Ar-H), 144.2 (Ar-H), 151.6, 166.9 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 171.8, 174.7; MS (C13H13N5O2S2): 358 [M + Na]+.
O), 171.8, 174.7; MS (C13H13N5O2S2): 358 [M + Na]+.
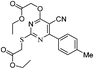
Ethyl 4-((ethoxycarbonyl)(mercapto)methylene)-7-amino-3,4-dihydro-2-phenylthieno[3,2-d]pyrimidine-6-carboxylate (14)
Pyrimidine (11a, 1 mmol), ethyl thioglycolate (2.1 mmol) and NaH (4 mmol) in dry THF (20 mL) were refluxed at 70–80 °C. After completion of the reaction, THF was removed under reduced pressure and residue was poured on cold water with stirring. The aqueous phase was neutralized with N/10 HCl (if required). The crude product obtained was filtered and purified by a silica gel column chromatography using ethyl acetate (1.0%) in hexane. Yellowish green solid, green fluorescence in long range UV, Rf: 0.55 (10% EtOAc in hexane); λmax/nm−1 (ε/dm−3 mol−1 cm−1): 203 (850), 272 (400), 357 (100); IR (KBr, νmax/cm−1): 3495 (NH), 3376 (NH), 1674 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); 1H NMR (CDCl3, 300 MHz, δ ppm): 1.38 (t, J = 7.2 Hz, 3H, CH3), 1.53 (t, J = 6.9 Hz, 3H, CH3), 1.66 (s, 1H, SH), 4.34 (q, J = 7.2 Hz, 2H, OCH2), 4.74 (q, J = 6.9 Hz, 2H, OCH2), 6.43 (bs, 2H, NH2), 7.45–7.47 (m, 3H, Ar-H), 8.44.8.47 (m, 2H, Ar-H); 13C NMR (CDCl3, 75 MHz, δ ppm): 14.8 (CH3), 14.9 (CH3), 60.7 (OCH2), 63.5 (OCH2), 94.8, 109.8, 128.8 (2 × Ar-H), 129.0, (2 × Ar-H), 131.4 (Ar-H), 137.4, 147.6, 163.0, 165.3, 165.5, 169.6; MS (C19H19N3O4S2): 455 [M + K − H]+.
O); 1H NMR (CDCl3, 300 MHz, δ ppm): 1.38 (t, J = 7.2 Hz, 3H, CH3), 1.53 (t, J = 6.9 Hz, 3H, CH3), 1.66 (s, 1H, SH), 4.34 (q, J = 7.2 Hz, 2H, OCH2), 4.74 (q, J = 6.9 Hz, 2H, OCH2), 6.43 (bs, 2H, NH2), 7.45–7.47 (m, 3H, Ar-H), 8.44.8.47 (m, 2H, Ar-H); 13C NMR (CDCl3, 75 MHz, δ ppm): 14.8 (CH3), 14.9 (CH3), 60.7 (OCH2), 63.5 (OCH2), 94.8, 109.8, 128.8 (2 × Ar-H), 129.0, (2 × Ar-H), 131.4 (Ar-H), 137.4, 147.6, 163.0, 165.3, 165.5, 169.6; MS (C19H19N3O4S2): 455 [M + K − H]+.
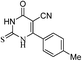
4-oxo-2-Thioxo-6-p-tolyl-1,2,3,4-tetrahydropyrimidine-5-carbonitrile (18)
Benzaldehyde (17, 5 mmol), methyl cyanoacetate (16, 5 mmol), thiourea (15, 5 mmol) and K2CO3 (5 mmol) in ethanol (25 mL) were refluxed at 120 °C. After completion of the reaction, ethanol was removed under reduced pressure and residue was poured on cold water with stirring. The aqueous phase was neutralized with N/10 HCl (if required). The crude product obtained was filtered and recrystallized with methanol. Yellow solid, Rf: 0.09 (dichloromethane); λmax/nm−1 (ε/dm−3 mol−1 cm−1): 220 (1300), 298 (1700); IR (KBr, νmax/cm−1): 3472 (NH), 3361 (NH), 2208 (CN), 1632 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); 1H NMR (DMSO-d6, 300 MHz, δ ppm): 2.50 (s, 3H, CH3), 7.31 (d, J = 7.8 Hz, 2H, Ar-H), 7.92 (d, J = 7.8 Hz, 2H, Ar-H), 12.99 (bh, 2H, NH); 13C NMR (DMSO-d6, 75 MHz, δ ppm): 22.1 (CH3), 103.4, 117.1 (CN), 130.7 (2 × Ar-H), 131.5 (2 × Ar-H), 144.8, 154.9, 162.2, 164.2, 177.5; MS (C12H9N3OS): 242 [M − H]+.
O); 1H NMR (DMSO-d6, 300 MHz, δ ppm): 2.50 (s, 3H, CH3), 7.31 (d, J = 7.8 Hz, 2H, Ar-H), 7.92 (d, J = 7.8 Hz, 2H, Ar-H), 12.99 (bh, 2H, NH); 13C NMR (DMSO-d6, 75 MHz, δ ppm): 22.1 (CH3), 103.4, 117.1 (CN), 130.7 (2 × Ar-H), 131.5 (2 × Ar-H), 144.8, 154.9, 162.2, 164.2, 177.5; MS (C12H9N3OS): 242 [M − H]+.
Synthesis of ethyl 2-(5-cyano-2-thioxo-6-p-tolyl-1,2-dihydro pyrimidin-4-yloxy)acetate (20) and ethyl 2-(5-cyano-2-(2-ethoxy-2-oxoethylthio)-6-p-tolylpyrimidin-4-yloxy)acetate (21)
In a 100 mL R.B. flask, 4-oxo-2-thioxo-6-p-tolyl-1,2,3,4-tetrahydropyrimidine-5-carbonitrile (18, 2 mmol) was dissolved in dimethylformamide (DMF, 15 mL) at 80 °C. After stirring for five minutes, anhydrous K2CO3 (2.1 mmol) and ethyl 2-bromoacetate (19, 2.0 mmol) were added in reaction mixture. The reaction mixture was stirred for 9 h. The reaction mixture was poured on cold water with vigorous stirring and neutralized it with acetic acid. The precipitate was filtered and dried. The crude was purified by column chromatography using silica gel (100–200 mesh) which yielded compound 20 (2.0% EtOAc in hexane) and 21 (2.5% EtOAc in hexane).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); 1H NMR (CDCl3, 300 MHz, δ ppm): 1.32 (t, J = 7.2 Hz, 3H, CH3), 2.46 (s, 3H, CH3), 4.27 (q, J = 7.2 Hz, 2H, OCH2), 4.76 (s, 2H, OCH2), 7.32 (d, J = 8.1 Hz, 2H, Ar-H), 7.92 (d, J = 8.4 Hz, 2H, Ar-H), 8.29 (s, 1H, NH); 13C NMR (CDCl3, 75 MHz, δ ppm): 14.4 (CH3), 22.2 (CH3), 62.1 (OCH2), 62.4 (OCH2), 100.9, 115.7 (CN), 129.1, 130.5 (2 × Ar-H), 131.8 (2 × Ar-H), 145.5, 156.5 (2 × Ar), 162.7, 167.2; MS (C16H15N3O3S): 330 [M + H]+.
O); 1H NMR (CDCl3, 300 MHz, δ ppm): 1.32 (t, J = 7.2 Hz, 3H, CH3), 2.46 (s, 3H, CH3), 4.27 (q, J = 7.2 Hz, 2H, OCH2), 4.76 (s, 2H, OCH2), 7.32 (d, J = 8.1 Hz, 2H, Ar-H), 7.92 (d, J = 8.4 Hz, 2H, Ar-H), 8.29 (s, 1H, NH); 13C NMR (CDCl3, 75 MHz, δ ppm): 14.4 (CH3), 22.2 (CH3), 62.1 (OCH2), 62.4 (OCH2), 100.9, 115.7 (CN), 129.1, 130.5 (2 × Ar-H), 131.8 (2 × Ar-H), 145.5, 156.5 (2 × Ar), 162.7, 167.2; MS (C16H15N3O3S): 330 [M + H]+.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); 1H NMR (CDCl3, 300 MHz, δ ppm): 1.24 (t, J = 7.2 Hz, 3H, CH3), 1.30 (t, J = 7.2 Hz, 3H, CH3), 2.42 (s, 3H, CH3), 3.96 (s, 2H, CH2), 4.19 (q, J = 7.2 Hz, 2H, OCH2), 4.27 (q, J = 7.2 Hz, 2H, SCH2), 5.01 (s, 2H, OCH2), 7.29 (d, J = 8.1 Hz, 2H, Ar-H), 7.94 (d, J = 8.4 Hz, 2H, Ar-H); 13C NMR (CDCl3, 75 MHz, δ ppm): 14.4 (CH3), 14.5 (CH3), 21.9 (CH3) 34.2 (SCH3), 62.1 (OCH2), 62.3 (OCH2), 64.2 (OCH2), 88.3, 114.7 (CN), 129.5 (2 × Ar-H), 129.8 (2 × Ar-H), 132.3 (Ar-H), 143.2, 167.3, 168.9, 169.2, 169.5, 173.4; MS (C20H21N3O5S): 416.2 [M + H]+, 438.3 [M + Na]+, 454.1 [M + K]+.
O); 1H NMR (CDCl3, 300 MHz, δ ppm): 1.24 (t, J = 7.2 Hz, 3H, CH3), 1.30 (t, J = 7.2 Hz, 3H, CH3), 2.42 (s, 3H, CH3), 3.96 (s, 2H, CH2), 4.19 (q, J = 7.2 Hz, 2H, OCH2), 4.27 (q, J = 7.2 Hz, 2H, SCH2), 5.01 (s, 2H, OCH2), 7.29 (d, J = 8.1 Hz, 2H, Ar-H), 7.94 (d, J = 8.4 Hz, 2H, Ar-H); 13C NMR (CDCl3, 75 MHz, δ ppm): 14.4 (CH3), 14.5 (CH3), 21.9 (CH3) 34.2 (SCH3), 62.1 (OCH2), 62.3 (OCH2), 64.2 (OCH2), 88.3, 114.7 (CN), 129.5 (2 × Ar-H), 129.8 (2 × Ar-H), 132.3 (Ar-H), 143.2, 167.3, 168.9, 169.2, 169.5, 173.4; MS (C20H21N3O5S): 416.2 [M + H]+, 438.3 [M + Na]+, 454.1 [M + K]+.
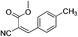
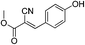
Synthesis of (E)-methyl 2-cyano-3-phenylacrylate (22)
Benzaldehyde (17, 5 mmol), methyl cyanoacetate (16, 3 mmol), thiourea (15, 3 mmol, in presence or in absent) and K2CO3 (3 mmol) in water (15 mL) were sake at room temperature 5 to 15 min. The aqueous phase was neutralized with N/10 HCl (if required). The precipitate was filtered as pure product.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); 1H NMR (CDCl3, 300 MHz, δ ppm): 2.41 (s, 3H, CH3), 3.91 (s, 3H, OCH3), 7.27 (d, J = 8.1 Hz, 2H, Ar-H), 7.89 (d, J = 8.4 Hz, 2H, Ar-H), 8.20 (s, 1H,
O); 1H NMR (CDCl3, 300 MHz, δ ppm): 2.41 (s, 3H, CH3), 3.91 (s, 3H, OCH3), 7.27 (d, J = 8.1 Hz, 2H, Ar-H), 7.89 (d, J = 8.4 Hz, 2H, Ar-H), 8.20 (s, 1H, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH); 13C NMR (CDCl3, 75 MHz, δ ppm): 22.2 (CH3), 53.6 (OCH3), 101.5 (
CH); 13C NMR (CDCl3, 75 MHz, δ ppm): 22.2 (CH3), 53.6 (OCH3), 101.5 (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 116.1 (CN), 129.2, 130.4 (2 × Ar-H), 131.6 (2 × Ar-H), 145.1, 155.6 (
C), 116.1 (CN), 129.2, 130.4 (2 × Ar-H), 131.6 (2 × Ar-H), 145.1, 155.6 (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 163.6 (C
CH), 163.6 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); MS (C12H11NO2): 224 [M + Na]+.
O); MS (C12H11NO2): 224 [M + Na]+.
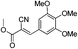
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); 1H NMR (CDCl3, 300 MHz, δ ppm): 3.26 (s, 6H, 2 × OCH3), 3.91 (s, 3H, OCH3), 3.92 (s, 3H, OCH3), 7.34 (s, 2H, Ar-H), 8.19 (s, 1H,
O); 1H NMR (CDCl3, 300 MHz, δ ppm): 3.26 (s, 6H, 2 × OCH3), 3.91 (s, 3H, OCH3), 3.92 (s, 3H, OCH3), 7.34 (s, 2H, Ar-H), 8.19 (s, 1H, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH); 13C NMR (CDCl3, 75 MHz, δ ppm): 52.3 (OCH3), 55.2 (2 × OCH3), 59.9 (OCH3), 99.7 (
CH); 13C NMR (CDCl3, 75 MHz, δ ppm): 52.3 (OCH3), 55.2 (2 × OCH3), 59.9 (OCH3), 99.7 (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 107.8 (2 × Ar-H), 114.9 (CN), 125.5, 141.7, 152.2 (2C), 154.1 (
C), 107.8 (2 × Ar-H), 114.9 (CN), 125.5, 141.7, 152.2 (2C), 154.1 (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 162.0 (C
CH), 162.0 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); MS (C14H15NO5): 278.2 (M + H)+, 300.2 [M + Na]+.
O); MS (C14H15NO5): 278.2 (M + H)+, 300.2 [M + Na]+.
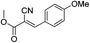
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); 1H NMR (CDCl3, 300 MHz, δ ppm): 3.87 (s, 3H, OCH3), 3.89 (s, 3H, OCH3), 6.97 (d, J = 9.0 Hz, 2H, Ar-H), 7.97 (d, J = 9.0 Hz, 2H, Ar-H), 8.15 (s, 1H,
O); 1H NMR (CDCl3, 300 MHz, δ ppm): 3.87 (s, 3H, OCH3), 3.89 (s, 3H, OCH3), 6.97 (d, J = 9.0 Hz, 2H, Ar-H), 7.97 (d, J = 9.0 Hz, 2H, Ar-H), 8.15 (s, 1H, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH); 13C NMR (CDCl3, 75 MHz, δ ppm): 53.5 (OCH3), 56.0 (OCH3), 99.3 (
CH); 13C NMR (CDCl3, 75 MHz, δ ppm): 53.5 (OCH3), 56.0 (OCH3), 99.3 (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 115.2 (2 × Ar-H), 116.5 (CN), 124.7, 134.0 (2 × Ar-H), 154.9 (
C), 115.2 (2 × Ar-H), 116.5 (CN), 124.7, 134.0 (2 × Ar-H), 154.9 (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 164.0 (C
CH), 164.0 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 164.2; MS (C12H11NO3): 218.2 [M + H]+, 240.1 [M + Na]+, 256.2 [M + K]+.
O), 164.2; MS (C12H11NO3): 218.2 [M + H]+, 240.1 [M + Na]+, 256.2 [M + K]+.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); 1H NMR (CDCl3, 300 MHz, δ ppm): 3.49 (h, 1H, OH), 3.89 (s, 3H, OCH3), 6.94 (d, J = 9.0 Hz, 2H, Ar-H), 7.90 (d, J = 9.0 Hz, 2H, Ar-H), 8.14 (s, 1H,
O); 1H NMR (CDCl3, 300 MHz, δ ppm): 3.49 (h, 1H, OH), 3.89 (s, 3H, OCH3), 6.94 (d, J = 9.0 Hz, 2H, Ar-H), 7.90 (d, J = 9.0 Hz, 2H, Ar-H), 8.14 (s, 1H, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH); 13C NMR (CDCl3, 75 MHz, δ ppm): 52.2 (OCH3), 96.3 (
CH); 13C NMR (CDCl3, 75 MHz, δ ppm): 52.2 (OCH3), 96.3 (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 115.6 (CN), 115.8 (2 × Ar-H), 122.0, 133.2 (2 × Ar-H), 154.2 (
C), 115.6 (CN), 115.8 (2 × Ar-H), 122.0, 133.2 (2 × Ar-H), 154.2 (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 162.3 (C
CH), 162.3 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 162.9; MS (C11H9NO3): 203 [M + H]+.
O), 162.9; MS (C11H9NO3): 203 [M + H]+.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); 1H NMR (CDCl3, 300 MHz, δ ppm): 3.80 (s, 3H, OCH3), 3.83 (s, 3H, OCH3), 4.62 (h, 1H, OH), 6.87 (d, J = 8.4 Hz, 1H, Ar-H), 7.27 (d, J = 8.4 Hz, 1H, Ar-H), 7.68 (s, 1H, Ar-H), 8.02 (s, 1H,
O); 1H NMR (CDCl3, 300 MHz, δ ppm): 3.80 (s, 3H, OCH3), 3.83 (s, 3H, OCH3), 4.62 (h, 1H, OH), 6.87 (d, J = 8.4 Hz, 1H, Ar-H), 7.27 (d, J = 8.4 Hz, 1H, Ar-H), 7.68 (s, 1H, Ar-H), 8.02 (s, 1H, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH); 13C NMR (CDCl3, 75 MHz, δ ppm): 53.4 (OCH3), 56.3 (OCH3), 97.9 (
CH); 13C NMR (CDCl3, 75 MHz, δ ppm): 53.4 (OCH3), 56.3 (OCH3), 97.9 (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 112.4 (Ar-H), 116.0 (Ar-H), 116.9 (CN), 123.9, 129.0 (Ar-H), 148.1, 152.7, 155.6 (
C), 112.4 (Ar-H), 116.0 (Ar-H), 116.9 (CN), 123.9, 129.0 (Ar-H), 148.1, 152.7, 155.6 (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 164.1 (C
CH), 164.1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); MS (C12H11NO4): 233 [M + H]+.
O); MS (C12H11NO4): 233 [M + H]+.
Acknowledgements
This work supported by DST, New Delhi, India for financial support as DST fast track young scientist fellowship [no SR/FT/CS-20/2011] to H.K.M. Authors are thankful to Director, CSIR-CIMAP for his kind support.Notes and references
- (a) M. Nett and C. Hertweck, J. Nat. Prod., 2011, 74, 2265 CrossRef CAS PubMed; (b) Y. Wang, J. Zheng, P. Liu, W. Wang and W. Zhu, Mar. Drugs, 2011, 9, 1368 CrossRef CAS PubMed; (c) H. Hara, T. Horiuchi, M. Saneyoshi and S. Nishimura, Biochem. Biophys. Res. Commun., 1970, 38, 305 CrossRef CAS; (d) I. M. Lagoja, Chem. Biodiversity, 2005, 2, 1 CrossRef CAS PubMed.
- (a) G. Goncalves, I. Tomaz, I. Correia, L. F. Veiros, M. M. C. A. Castro, F. Avecilla, L. Palacio, M. Maestro, T. Kiss, T. Jakusch, M. H. V. Garcia and J. C. Pessoa, Dalton Tran., 2013, 42, 11841 RSC; (b) A. Paudel, K. Kaneko, A. Watanabe, M. Shigeki, K. Motomu, H. Hamamoto and K. Sekimizu, J. Antibiot., 2013, 66, 663 CrossRef CAS PubMed; (c) X. Zhang, P. W. Glunz, W. Jiang, A. Schmitt, M. Newman, F. A. Barbera, J. M. Bozarth, A. R. Rendina, A. Wei, X. Wen, K. A. Rossi, J. M. Luettgen, P. C. Wong, R. M. Knabb, R. R. Wexler and E. S. Priestley, Bioorg. Med. Chem. Lett., 2013, 23, 1604 CrossRef CAS PubMed; (d) X.-J. Zhang, L. H. Lu, R.-R. Wang, Y.-P. Wang, R.-H. Luo, C. C. Lai, L.-M. Yang, Y.-P. He and Y. T. Zheng, PLoS One, 2013, 8(11), e81489, DOI:10.1371/journal.pone.0081489; (e) A. Seiler and U. Mueller, Eur. Pat., 136976 A2 19850410, 1985; (f) G. Y. Lesher, B. Singh and Z. E. Mielens, J. Med. Chem., 1982, 25, 837 CrossRef CAS; (g) R. Dudhe, P. K. Sharma, P. Verma and A. J. Chudhary, Adv. Sci. Res., 2011, 2, 10 CAS; (h) M. Amir, S. A. Javed and H. Kumar, Int. J. Pharma Sci. Res., 2007, 69, 337 CAS; (i) E. D. Clercq and A. Holy, Nat. Rev. Drug Discovery, 2005, 4, 928 CrossRef PubMed; (j) J. K. Gupta, A. Chaudhary, R. Dudhe, K. Varuna, P. K. Sharma and P. K. Verma, Int. J. Pharma Sci. Res., 2010, 1, 34 CAS; (k) H. K. Maurya, R. Verma, A. Saba, S. Pandey, V. Pathak, S. Sharma, K. K. Srivastava, A. S. Negi and A. Gupa, Bioorg. Med. Chem. Lett., 2013, 23, 5844 CrossRef CAS PubMed; (l) N. Agarwal, P. Srivastava, S. K. Raghuwanshi, D. N. Upadhyay, S. Sinha, P. K. Shukla and V. J. Ram, Bioorg. Med. Chem., 2002, 10, 869 CrossRef CAS.
- P. Pacher, A. Nivorozhkin and C. Szabo, Pharmacol. Rev., 2006, 58, 87 CrossRef CAS PubMed.
- R. R. Jetti, M. Jonnalagadda, C. K. Raval and D. Datta, WO Pat., 2011024192-A2; WO2011024192-A3; IN200901765-I4, 2011.
- K. Lin, Y. Bi, D. Zhu and Q. You, Faming Zhuanli Shenqing, CN 103169710 A 20130626, 2013.
- K. S. Jain, T. S. Chitre, P. B. Miniyar, M. K. Kathiravan, V. S. Bendre, V. S. Veer, C. J. Shahane and S. R. Shishoo, Current Sci., 2006, 90, 793 CAS.
- T. P. Selvam, C. R. James, P. V. Dniandev and S. K. Valzita, Res. Pharm., 2012, 2, 1 Search PubMed.
- (a) C. I. Herrerias, X. Yao, Z. Li and C.-J. Li, Chem. Rev., 2007, 107, 2546 CrossRef CAS PubMed; (b) H. C. Hailes, Org. Process Res. Dev., 2007, 11, 114 CrossRef CAS.
- (a) L. El Kaim, L. Grimaud and S. Wagschal, Chem. Commun., 2011, 47, 1887 RSC; (b) M. K. Dahlgren, A. B. Garcia, A. A. Hare, J. Tirado-Rives, L. Leng, R. Bucala and W. L. Jorgensen, J. Med. Chem., 2012, 55, 10148 CrossRef CAS PubMed; (c) Z. Liu, D. Li, S. Li, D. Bai, X. He and Y. Hu, Tetrahedron, 2007, 63, 1931 CrossRef CAS PubMed; (d) Y. Hu, Z. Liu, D. Li, S. Li, X. He and D. Bai, Faming Zhuanli Shenqing, 101121718, 2008; (e) G. Y. Lesher, B. Singh and Z. E. Mielens, J. Med. Chem., 1982, 25, 837 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2014 |