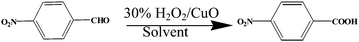Bio-derived CuO nanocatalyst for oxidation of aldehyde: a greener approach†
Chandan Tamuly*a,
Indranirekha Saikiaa,
Moushumi Hazarikaa and
Manash R. Dasb
aNatural Product Chemistry Section, CSIR-North East Institute of Science and Technology, Branch Itanagar, Arunachal Pradesh-791110, India. E-mail: c.tamuly@gmail.com; Fax: +91 3602244220; Tel: +91 3602244220
bMaterial Science Division, CSIR-North East Institute of Science and Technology, Jorhat, Assam-785006, India
First published on 29th April 2014
Abstract
Eco-friendly synthesis of hierarchical CuO nanoparticles using the peel of Musa balbisiana and its application as a nanocatalyst in oxidation of aldehyde to the corresponding carboxylic acid is reported here. CuO nanoparticles were characterized by using XRD, XPS, SEM, TEM and PL techniques. In XRD analysis, significant peaks appeared at 18.2, 24.6, 33.3, 34.9, 35.5, 38.6 and 42.3. The SEM images indicate the formation of a micro flower hierarchical CuO architecture. The hierarchical CuO architecture is found to be made up of 2D nanosheets as building blocks, which were self assembled to form micro flower like assemblies. The synthesized CuO nanoparticles are efficiently utilised in the oxidation of aldehyde to the corresponding carboxylic acid in the presence of 30% H2O2 with high yields. The utilization of the CuO nanocatalyst in the oxidation reaction in environmental friendly conditions is the novelty in this study.
Introduction
Cupric oxide (CuO) has received much attention for the applications in fields including catalysis, batteries, magnetic storage media, solar energy conversion, gas sensing, field emission transistors, energy conversion and storage, electronics, sensors and environmental science.1–4 The prospect of potential applications of CuO nanostructures has led to substantial research and development effort to form various types of nanostructures. So far, a variety of CuO nanostructures including nanoparticles, nanoneedles, nanowhiskers, nanowires, nanobelts and nanosheets have been obtained successfully by various methods namely alcothermal,5 hydrothermal,6 electrodeposition,7 thermal oxidation,8 reverse micelle9 and microwave synthesis.10 It is still a challenge to develop a simple, rapid, eco-friendly, easy to control and energy-efficient method for preparation of CuO nanostructures with suitable catalytic activity.Biosynthesis of metal oxide nanoparticles using natural sources like plants and microorganism is becoming an emerging area of research in nano-biotechnology due to its clean, nontoxic, and environmentally friendly nature. Generally, the preparation of CuO nanoparticles uses a variety of methods including sol–gel, quick precipitation, sonochemical, electrochemical, solid state reaction, alcohothermal synthesis, microwave irradiation, and liquid–liquid interface techniques involving organic solvents and harsh reducing agents.11 Therefore, there is a need to develop newer eco friendly processes for the synthesis of CuO nano particles and it is a challenging task to find a convenient, mild, nontoxic, natural product to produce metal nanoparticles in an aqueous environment. Several studies have been made in this regard in recent years. The green synthesis of metallic nanoparticles includes use of biological agents such as bacteria, fungi, actinomycetes, yeast and plants12 has been studied by several research groups. The microorganism like Streptomyces sp.,13 Escherichia coli,14 fungi Penicillium aurantiogriseum, Penicillium citrinum and Penicillium waksmanii,15 Morganella sp.,16 Phormidium cyanobacterium17 etc. was extensively used in biosynthesis of CuO nanoparticles. Biosynthesis of CuO nanoparticles by plants is currently in under development process. Lee et al. used Magnolia kobus leaf extract for the synthesis of Cu nano particles.18 Gopalakrishnan reported the synthesis of Cu nanoparticles from Fehling's solution using Tridax procumbens leaf extract.19 Vellora et al. used gum karaya as a biotemplate for the biosynthesis of CuO nano particles.20 Looking towards the unavailability of such methods, we were intended to develop an alternative procedure for the biosynthesis of CuO nanoparticles using the peel of Musa balbisiana. This method is more valuable since production of nanoparticles by chemical method may lead to absorption of harsh chemicals on the surfaces of nanoparticles raising the toxicity issue. Our procedure is environmentally benign technique for production of well characterized nanoparticles without use of harsh, toxic and expensive chemicals. Furthermore, synthesis of metal nano particles using plant materials is very cost effective and therefore can be used as an economic and valuable alternative for the large scale production of CuO nanoparticles. Again in our case the waste materials i.e. the peel of Musa balbisiana was used which is another advantage of this method.
The transformation of aldehydes to carboxylic acids is an important and most fundamental reaction in organic chemistry. The oxidation of aldehydes is of interest owing to their potential in organic synthesis and industrial manufacturing.21 Most of the oxidation reactions were carried out by the use of varieties of oxidizing agent like potassium permanganate, chromic acid, bromine, nitric acid, Jones reagent, H2O2 etc.22 However, H2O2 is regarded as environment friendly oxidant which can be used in both laboratory and industrial scale. Since the only degradation product of its use is water, and thus it has played a large role in environmentally friendly methods in the chemical industry.23 The aqueous H2O2 has been considered to have a weak ability to oxidize aldehydes. There are numerous approaches described in the literature for the synthesis of carboxylic acids.24,25 These protocols suffer from shortcomings such as large waste production, higher reaction temperature, prolonged reaction time, low yields, harsh conditions, undesirable by products, toxicity, low recovery, and reusability of the catalyst. Therefore, to overcome these drawbacks afford has been done for a convenient protocol of synthesis carboxylic acid from corresponding aldehyde.
Here we reported eco friendly, simple, efficient method for synthesis and characterization of hierarchical CuO nanoparticles by using the peel of Musa balbisiana (alkaline precursors). In order to demonstrate the application of our synthesized nano particle in organic synthesis; we are reporting here the oxidation of aldehydes to acids using H2O2 as oxidant in presence of CuO nano particle. It is a convenient, very simple, efficient protocol of synthesis carboxylic acid from corresponding aldehyde from the green chemistry point of view which is suitable for medium and large scale reactions in oxidation of aldehyde to corresponding carboxylic acid.
Results and discussion
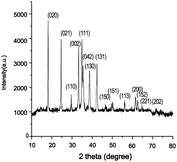
In the present investigation, an eco-friendly, cost effective, green, simple approach in synthesis of hierarchical CuO nanoparticles by using peel of Musa balbisiana is demonstrated. The peel of the plant burnt in muffle at 500 °C for 1 h and the ash was filtered. 1 kg of the ash contained 233.60 g of K+, 2.00 g of Na+, 161.40 g of CO32− and 6.62 g of Cl−, when prepared from peel of Musa balbisiana.26 CuSO4·5H2O react with the alkaline precursors like K+, Na+, CO32− etc. to formed Cu(OH)2 which further calcinations at temperature 500 °C for 1 h to formed CuO nanoparticles. These ions may be responsible for formation of Cu(OH)2, which further undergo in formation of CuO nanoparticles by thermal process [ESI, Scheme 1†].The formation of CuO nanoparticles was confirmed by using XRD analysis. In XRD analysis, the planes (020), (021), (110), (002), (111), (042), (130), (131), (150), (151), (113), (200), (152), (221) and (202) indicates the formation of monoclinic crystallite without having any peak due to the possible Cu2O and Cu(OH)2 impurity and well arrange in specific orientation.27 The significant 2θ values appeared at 18.2, 24.6, 33.3, 34.9, 35.5, 38.6, 42.3 corresponds to (020), (021), (002), (111), (042), (138) and (131) planes respectively (Fig. 1A). The corresponding d values are 4.87, 3.61, 2.68, 2.58, 2.52, 2.32 and 2.13 Å respectively. These are very close to those in the JCPDS File no. 5-0661. The result is similar as reported data.28
The formation of CuO nanoparticles was further confirmed by using FT-IR analysis. The FT-IR spectrum of CuO nano pellets is shown (ESI, Fig. S1†). Weak band at 2361 cm−1 assigned to carboxylic group (COO−) vibration. Peak at 1629 cm−1 assigned to amide (COO−) while the very intense peak positioned at 1112 cm−1 revealed the presence of (O–H) stretching for alkyl. These characteristic peaks indicated the presence of very small amount of protein/amino acid molecules along with CuO nanoparticles. The characteristic peaks of CuO positioned from 984 cm−1 to 450 cm−1. The peaks positioned at around 640, 606 cm−1 and 530 cm−1 corresponding to the characteristic stretching vibrations of Cu–O bond in the monoclinic CuO. The peak observed at 530 and 606 cm−1 due to Cu–O stretching along the [−202] direction and 450 cm−1 from Cu–O stretching along the [202] direction in CuO crystal.27
The Fig. 2A and B shows the XPS spectra of CuO nanoparticles. The spectrum obtained was calibrated binding energy (BE) at 284.5 eV for a C 1s electron. The XPS BE at 941 and 961 eV corresponds to Cu 2p3/2 and Cu 2p1/2 respectively which is in agreement with reported data.29 In addition to these peaks, there is observation of other peak at 951 eV correspond to the shake-up satellite peaks of Cu (2p3/2).30 The BE at 538 eV corresponds to O 1s of CuO nanoparticles. It is strongly supported by reported data.31 The XPS analysis reveal that no Cu2O and Cu(OH)2 impurities are present within the sample.
The SEM images indicate the formation of micro flower like hierarchical CuO nanoparticles. Fig. 3A and B shows the SEM images of CuO products synthesized using peel of Musa balbisiana (alkaline precursor). It can be observed that the peel of Musa balbisiana have substantial impact on the growth of CuO nanostructures. SEM images of sample synthesized using peel of Musa balbisiana as alkaline precursor showed the formation of 3D flower-like hierarchical CuO architecture with an average diameter of about 200–400 nm. Higher magnification shows that the flower-like hierarchical micro flowers are composed of 2D nanosheet subunits as building blocks, which were self-organized to form micro flower assemblies. The HR-TEM analysis results showed the formation of micro flower like cluster of CuO nanostructure. The nanoparticles overlap each other which strongly support the formation of flower like nanostructure along with spherical and oval shape (Fig. 3C and D). The size of CuO nanoparticles was found in the range of 10.0 ± 0.2–40.0 ± 1.3 nm. The average size of the particles is 23.5 ± 0.8 nm. The difference between the two atomic layers is 0.16 nm. Further, the EDX spectra clearly indicated the formation of CuO nanoparticles [ESI, Fig. S2†]. The element C, O, K, Cl etc. may be due to presence of minerals with the extract of Musa balbisiana.
In photoluminescence spectra, two emission peaks are observed at 398 nm (violet), 470 nm (blue) was observed (Fig. 4). The first one corresponds to the band-edge emission and second one is due to artefact.32
Initial attempts to optimize the reaction conditions for the oxidation of aldehyde to the corresponding carboxylic acid were done using 4-nitrobenzaldehyde as the substrate in presence of different solvents and 2 mol% of CuO catalyst (Table 1). The reaction was observed by using different solvent like acetonitrile, ethyl acetate, chloroform, DMF, toluene, ethanol, methanol and DMSO. From the Table 1, it was observed that acetonitrile was best solvent in oxidation of 4-nitro benzaldehyde to 4-nitrocarboxylic acid when 2 mol% CuO nanocatalyst and 10 equiv. 30% H2O2 use as oxidant at 60 °C temperature. In acetonitrile, 95% yield was found within 1 h of reaction time. In case of other solvent the product was formed in longer period of time with comparatively low yield.
| Entry | Solvent | Timea (h) | Yieldb (%) |
|---|---|---|---|
| a Reactions performed at 60 °C and monitored using TLC until all the aldehyde was found to have been consumed.b Isolated yield after column chromatography of the crude product with 2% standard deviation. | |||
| 1 | Ethyl acetate | 2 | 75 |
| 2 | Acetonitrile | 1 | 95 |
| 3 | Toluene | 3 | 80 |
| 4 | Chloroform | 11 | 72 |
| 5 | Methanol | 10 | 83 |
| 6 | Ethanol | 11 | 85 |
| 7 | THF | 10 | 84 |
| 8 | DMSO | 12 | 77 |
| 9 | DMF | 13 | 79 |
The catalytic application of the synthesized CuO nanoparticles towards the oxidation of aldehyde has been investigated. A variety of aldehydes are smoothly oxidized to their corresponding carboxylic acids with high yields. During the reaction, other functional groups such as nitro, dinitro and chloro remain intact (Table 2).
| Entry | Aldehyde | Acid | Timea (h) | Yieldb (%) | TOFc (h−1) |
|---|---|---|---|---|---|
| a Reactions performed at 60 °C and monitored using TLC until all the aldehyde was found to have been consumed.b Isolated yield after column chromatography of the crude product with 2% standard deviation.c TOF: turn over frequency. | |||||
| 1 |  |
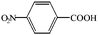 |
1.00 | 95 | 475.0 |
| 2 |  |
 |
2.00 | 87 | 217.5 |
| 3 |  |
 |
2.50 | 85 | 170.0 |
| 4 |  |
 |
5.00 | 82 | 82.0 |
| 5 |  |
 |
3.50 | 75 | 107.1 |
The progress of the oxidation reaction was monitored by silica-gel TLC. Upon completion of the reaction, the product was separated by silica-gel column chromatography using an appropriate eluent system. The isolated compounds were purified by 1H NMR, 13C NMR, FT-IR, GC-MS analysis. These data of all the products were comparable with the commercialized compounds. As shown in Table 2, all of the aldehyde afforded their corresponding carboxylic acid in excellent yields. The spectroscopic data of all isolated compounds is reported (ESI, Scheme 2†). The effect of electron donating group and electron withdrawing group on the benzaldehyde substrates cannot be generalized with respect to reaction time.33,34 It has been observed that the more substituted phenyl groups took longer time for completion of reaction.35 Our protocol provides a milder and facile methodology for oxidation reaction that is useful for organic synthesis.
Since, we have tested the reusability of CuO catalyst in the oxidation of 4-nitrobenzyldehyde. It is believed that CuO catalyst can be reused for this oxidation reaction as well. In oxidation of 4-nitrobenzyldehyde to 4-nitrobenzoic acid, the activity of catalyst was observed five times.
The CuO catalyst was recovered by simple filtration and was washed with hot water/ethanol to remove any absorbed products. The catalyst was reused without obvious loss of their catalytic activity, up to five cycles. Recyclability of the catalyst is shown in Fig. 5. It was confirmed that efficiency remain almost same after five times recycle of catalyst (1st recycle 95%, 2nd recycle 94% and 3rd recycle 92%, 4th recycle 91% and 5th recycle 90% 4-nitrobenzoic acid was obtained). It was further confirmed by using XRD and TEM analysis after 5th recycle [Fig. S3 and S4†].
The catalytic performance of the CuO nanocatalyst is compared with earlier reported results (ESI, Table S1†). Among all the reported catalyst, the CuO nanoparticles using 30% H2O2 as the oxidizing agent showed highest yield of 4-nitrobenzoic acid. TEM and XRD investigation also showed that the activity, morphology and size distribution of the CuO nanocatalyst remain unchanged after use of 5 times in catalytic reaction.
This is the first report on synthesis of bio-derived CuO nanoparticles by using peel of Musa balbisiana which is a waste materials having suitable catalytic activity. Moreover, this waste material is easily available and has also some medicinal value.26 This is the novelty in our study. This is one of the steps of utilization of waste material for value addition. Preparation of 3D flower-like hierarchical CuO architecture by with controlling the size (<40 nm) by using very simple eco-friendly method is another unique nature in our study.
Therefore it can be concluded that we have achieved a remarkable method for the oxidation of various aldehydes to their corresponding carboxylic acids using CuO nano catalyst, prepared from the peel of Musa balbisiana. This reaction is a room temperature reaction which gives a very good yield with a reasonable time period. The use of H2O2 is also promising for environmental perspectives.
The reaction is anticipated to follow an electron transfer mechanism where Cu(II) is reduced to Cu(I), accompanying the oxidation of aldehyde. The reduced Cu(I) is again oxidized to Cu(II) by the oxidant. Aqueous H2O2 is a eco-friendly, safe oxidant which produces no hazardous by product. Although its ability to oxidize aldehyde has been ignored, it is very useful for this purpose and found significant yield in mild reaction condition. Changing current practices to a process using this environmentally friendly, green oxidant is highly desirable.
Conclusions
It is very simple eco-friendly process of synthesis of CuO nanoparticles by using peel of Musa balbisiana. CuO flower-shaped hierarchical architectures were obtained by using the peel of Musa balbisiana (alkaline precursors). Owing to its great chemical flexibility and synthetic tenability, the present route provides a simple and green pathway of synthesizing 3D CuO nanostructures. Moreover, we have developed a simple, efficient, chemo selective and inexpensive catalytic method for the oxidation of aldehyde to carboxylic acid using CuO nanocatalyst and 30% H2O2 with high yield product. The CuO nanocatalyst was found to be highly active and could be recycled for five consecutive runs without significant loss of catalytic activity.Acknowledgements
The authors thank Director, CSIR-North East Institute of Science & Technology, Jorhat, Assam for valuable advice. The authors also thank SAIF, Shillong for TEM & SEM analysis and Dr K. R. Patil, NCL Pune for XPS analysis. The authors thank SEED Division, DST New Delhi for financial support.References
- (a) P. Poizot, S. Laruelle, S. Grugeon, L. Dupont and J. M. Tarascon, Nature, 2000, 407, 496–498 CrossRef CAS PubMed; (b) R. V. Kumar, Y. Diamant and A. Gedanken, Chem. Mater., 2000, 12, 2301–2305 CrossRef CAS.
- A. Chowdhuri, V. Gupta, K. Sreenivas, R. Kumar, S. Mozumdar and P. K. Patanjali, Appl. Phys. Lett., 2004, 84, 1180–1182 CrossRef CAS PubMed.
- J. Chen, S. Deng, N. Xu, W. Zhang, X. Wen and S. Yang, Appl. Phys. Lett., 2003, 83, 746–748 CrossRef CAS PubMed.
- J. T. Zhang, J. F. Liu, Q. Peng, X. Wang and Y. D. Li, Chem. Mater., 2006, 18, 867–871 CrossRef CAS.
- A. El-Trass, H. ElShamy, I. El-Mehaseeb and M. El-Kemary, Appl. Surf. Sci., 2012, 258, 2997–3001 CrossRef CAS PubMed.
- M. A. Dar, Y. S. Kim, W. B. Kim, J. M. Sohn and H. S. Shin, Appl. Surf. Sci., 2008, 254, 7477–7481 CrossRef CAS PubMed.
- N. Mukherjee, B. Show, S. K. Maji, U. Madhu, S. K. Bhar, B. C. Mitra, G. G. Khan and A. Mondal, Mater. Lett., 2011, 65, 3248–3250 CrossRef CAS PubMed.
- M. Kaur, K. P. Muthe, S. K. Despande, S. Choudhury, J. B. Singh, N. Verma, S. K. Gupta and J. V. Yakhmi, J. Cryst. Growth, 2006, 289, 670–675 CrossRef CAS PubMed.
- M. Yuasa, T. Masaki, T. Kida, K. Shimanoe and N. Yamazoe, Sens. Actuators, B, 2009, 136, 99–104 CrossRef CAS PubMed.
- J. Zhu and X. Qian, J. Solid State Chem., 2010, 183, 1632–1639 CrossRef CAS PubMed.
- M. Suleiman, M. Mousa, A. Hussein, B. Hammouti, T. B. Hadda and I. Warad, J. Mater. Environ. Sci., 2013, 4, 792–797 CAS.
- R. Varshney, S. Bhadauria and M. S. Gaur, Nano Biomed. Eng., 2012, 4, 99–106 CAS.
- R. Usha, E. Prabu, M. Palaniswamy, C. K. Venil and k. R. Rajendran, Global J. Biotechnol. Biochem., 2010, 5, 153–160 CAS.
- A. V. Singh, R. Patil, A. Anand, P. Milani and W. N. Gade, Curr. Nanosci., 2010, 6, 365–369 CrossRef CAS.
- S. Honary, H. Barabadi, E. Gharaei-Fathabad and F. Naghibi, Dig. J. Nanomater. Bios., 2012, 7, 999–1005 Search PubMed.
- R. Ramanathan, S. K. Bhargava and V. Bansal, Chemeca 2011: Engineering a Better World, Sydney Hilton Hotel, NSW, Australia, September 2011, pp. 18–21 Search PubMed.
- A. Rahman1, A. Ismail, D. Jumbianti, S. Magdalena and H. Sudrajat, Indian J. Chem., 2009, 9, 355–360 Search PubMed.
- H.-J. Lee, J. Y. Song and B. S. Kim, J. Chem. Technol. Biotechnol., 2013, 88, 1971–1977 CAS.
- K. Gopalakrishnan, C. Ramesh, V. Ragunathan and M. Thamilselvan, Dig J Nanomater Bios., 2012, 7, 833–839 Search PubMed.
- V. Vellora, T. Padil and M. Cerník, Int. J. Nanomed., 2013, 8, 889–898 Search PubMed.
- (a) G. J. Hollingworth, in Comprehensive Organic Functional Group Transformations, ed. A. R. Katritzky, O. Meth-Cohn, C. W. Rees and G. Pattenden, Elsevier Science, Oxford, 1995, vol. 5, p. 23 Search PubMed; (b) M. Hudlicky, Oxidations in Organic Chemistry, ACS Monograph Series 186, American Chemical Society, Washington, DC, 1990, p. 174 Search PubMed.
- R. C. Larock, Comprehensive Organic Transformations: a Guide to Functional Group Preparations, Wiley-VCH, New York, 2nd edn, 1999, p. 1653 Search PubMed.
- J. M. Campos-Martin, G. Blanco-Brieva and J. L. G. Fierro, Angew. Chem., Int. Ed., 2006, 45, 6962–6984 CrossRef CAS PubMed.
- M. Matsumoto, H. Kobayashi and Y. Hotta, J. Org. Chem., 1984, 49, 4740–4741 CrossRef CAS.
- L. Rémi, L. Pierre, L. Xiao and H. Catherine, European Patent, 0424 242 A2, 1991.
- D. C. Deka and N. N. Talukdar, Indian Journal of Traditional Knowledge, 2007, 6(10), 72–78 Search PubMed.
- N. V. Suramwar, S. R. Thakare and N. T. Khaty, Int. J. Nano Dimens., 2012, 3(1), 75–80 Search PubMed.
- K. Kannaki, P. S. Ramesh and D. Geetha, Int. J. Sci. Eng. Res., 2012, 3(9), 1–4 Search PubMed.
- M. A. Das, S. H. Nam, Y. S. Kim and W. B. Kim, J. Solid State Electrochem., 2010, 14, 1719–1726 CrossRef.
- K. Krishnamoorthy and S. J. Kim, Mater. Res. Bull., 2013, 48, 3136–3139 CrossRef CAS PubMed.
- C. H. Yoo and T. W. Kim, J. Ceram. Process. Res., 2011, 12(5), 606–609 Search PubMed.
- R. S. Ningthoujam, V. Sudarsan and S. K. Kulshreshtha, J. Lumin., 2007, 127, 747–756 CrossRef CAS PubMed.
- K. Sato, M. Hyodo, J. Takagi, M. Aoki and R. Noyori, Tetrahedron Lett., 2000, 41, 1439–1442 CrossRef CAS.
- G.-J. T. Brink, J. M. Vis, I. W. C. E. Arends and R. A. Sheldon, Tetrahedron, 2002, 58, 3977–3983 CrossRef.
- D. Chakraborty, C. Majumder and P. Malik, Appl. Organomet. Chem., 2001, 25, 487–490 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra01683a |
| This journal is © The Royal Society of Chemistry 2014 |