Efficient energy storage capabilities promoted by hierarchical MnCo2O4 nanowire-based architectures†
Abstract
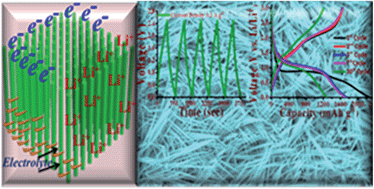
A facile, two-step hydrothermal/calcination approach to grow uniform MnCo2O4 nanowires (NWs) on a binder- and conductive-agent-free nickel foam substrate has been demonstrated. The hierarchical MnCo2O4 NW-based architectures on a conductive substrate allow for enhanced electrolyte transport and charge transfer towards/from the MnCo2O4 NWs' surfaces with large numbers of electroactive sites. In addition, the direct growth and attachment of the MnCo2O4 NWs on the supporting conductive substrates provide much reduced contact resistances and efficient charge transfer. These excellent features allow the use of MnCo2O4 NWs as a lithium-ion battery electrode, with high capacity at a current density of 200 mA h g−1, as well as a very good cycling stability. Moreover, these MnCo2O4 NWs have also demonstrated a high capacitive behavior for symmetric supercapacitor application, indicating the potential application of MnCo2O4 NW high-performance energy-storage devices.

 Please wait while we load your content...
Please wait while we load your content...