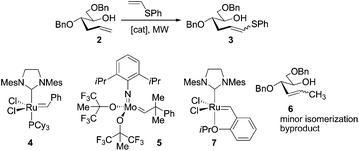
Ruthenium-catalyzed cross-metathesis with electron-rich phenyl vinyl sulfide enables access to 2,3-dideoxy-D-ribopyranose ring system donors†
Omar Boutureira*,
M. Isabel Matheu,
Yolanda Díaz and
Sergio Castillón
Departament de Química Analítica i Química Orgànica, Universitat Rovira i Virgili, C/Marcel·lí Domingo s/n, 43007 Tarragona, Spain. E-mail: omar.boutureira@urv.cat; Fax: +34 977558446; Tel: +34 977558288
First published on 16th April 2014
Abstract
2,3-Dideoxy-D-ribopyranose units are important ring systems found in nature. Herein, we develop a metal-mediated strategy to form this important scaffold featuring a cross-metathesis reaction of the corresponding sugar-derived hydroxyalkene with electron-rich phenyl vinyl sulfide using commercially available ruthenium-catalysts under microwave irradiation as a key step. The final 2,3-dideoxyhexopyranose ring is generated in a single step upon 6-endo electrophilic cyclization.
Introduction
2,3-Dideoxy- and 2,3,6-trideoxyhexoses are carbohydrate ring systems found in a variety of natural products.1 These structures are primary constituents of the oligosaccharide side chains of various antibiotics such as kigamicins,2 landomycins,3 urdamycins3,4 and amicetin,5 among others. Moreover, synthetic 2,3-dideoxy-D-ribopyranosyl nucleoside antibiotics have shown promising antitumor and antiviral activities and also constitute the repeating unit of unnatural hexopyranosyl-(6′ → 4′)-oligonucleotide systems6 (Fig. 1). | ||
| Fig. 1 Representative examples of compounds containing 2,3-dideoxy- and 2,3,6-trideoxyhexose ring systems. | ||
Previous strategies for the preparation of such structures are mainly limited to classical carbohydrate methods (from D-glucal), which typically suffer from long linear and operationally tedious sequences.1,7 Metal-mediated protocols have recently emerged as attractive alternatives because they enable straightforward access to key sugar intermediates and rare building blocks using a reduced number of steps.8 As such, olefin cross-metathesis (CM) represents a versatile, powerful metal-mediated C–C bond forming process for the construction of complex carbohydrate-based products.9 A number of novel applications have become possible due to major advances in catalyst design that led to high yielding transformations, under mild conditions and remarkably, in the presence of a variety of functional groups that were originally detrimental for a productive reaction.10 For example, by using allyl chalcogens11 as “forbidden”-atom-containing reactive handles, new and exciting applications such as chemical protein modifications are now accessible,12 hence representing the renaissance of such groups in catalysis. However, despite all this progress, CM reactions with electron-rich vinyl olefins13 remains an underrepresented area of olefin metathesis when compared to other twin systems such as ring-opening cross-metathesis (ROCM),14 ring-closing metathesis (RCM)15 and enyne cross-metathesis (EYCM).16 To the best of our knowledge, only a few examples of Ru-CM reactions involving electron-rich vinyl sulfides with model vinyl chlorides17 and silanes18 have been described to date, yet the use of such procedures for the preparation of more advanced, synthetically challenging systems remains largely unexplored. This reduced and sometimes non-existent reactivity has been attributed to the formation of relatively unreactive Fischer carbenes, which either rapidly decompose or fail to react further.19 These findings encouraged us to demonstrate that this particularly challenging transformation can be applied to the construction of an important 2,3-dideoxyhexopyranosyl ring system. Thus, a flexible strategy was envisaged starting from 2-deoxy-D-ribofuranose and featuring a CM reaction of the corresponding sugar-derived hydroxyalkene with electron-rich phenyl vinyl sulfide using commercially available Ru-catalysts under microwave (MW) irradiation as a key step (Scheme 1).
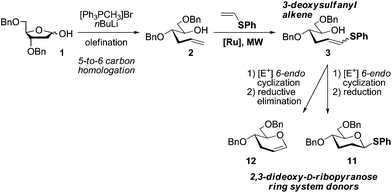 | ||
| Scheme 1 Proposed strategy for the preparation of 2,3-dideoxy-D-ribopyranose ring system donors 11 and 12 using a CM reaction with electron-rich phenyl vinyl sulfide as a key step. | ||
Indeed, this transformation will enable the concise synthesis of key 3-deoxysulfanyl alkene intermediate 3 from readily available starting materials that will be further elaborated to the expected 2,3-dideoxy ring system donors 11 (a ready-to-use 1-thioglycosyl donor) and 12 in very few steps.
Results and discussion
The first step of the proposed route involves a five-to-six carbon homologation of 2-deoxy-D-ribofuranose 1 to afford 2 using a reported Wittig olefination.20 We next focused on the synthesis of 3-deoxysulfanyl alkene intermediate 3 using the aforementioned CM of 2-deoxysugar hydroxyalkene 2 and phenyl vinyl sulfide as a key step (Table 1).| Entry | Catalyst (mol%) | Solvent | T (°C) | t (h) | Distributionb (%) | Z/E ratiob of 3 | |
|---|---|---|---|---|---|---|---|
| 2 | 3 | ||||||
a General conditions: a solution of phenyl vinyl sulfide (5 equiv.), catalyst (20 mol%) and 2 (1 equiv.) in dry and degassed solvent (0.5 M) was microwave irradiated in a sealed tube using a CEM-Discover™ single-mode synthesizer (temperature control using an external surface sensor, fixed hold time off, normal absorption mode) unless otherwise indicated.b Determined by 1H NMR.c Thermal heating under open vessel reflux conditions.d Prolonged reaction times did not increased the conversion.e Isolated yield after two consecutive reaction cycles (see Experimental section for details).f Added in two portions.g Variable amounts of isomerization byproduct 6 (10–28% and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 Z/E ratio) were also detected.h 2,6-Dichloro-1,4-benzoquinone (10 mol%) was added as an additive. DCB = 1,2-dichlorobenzene. 5 Z/E ratio) were also detected.h 2,6-Dichloro-1,4-benzoquinone (10 mol%) was added as an additive. DCB = 1,2-dichlorobenzene. |
|||||||
| 1c | 4 (20) | CH2Cl2 | 40 | 20 | 100 | — | — |
| 2c | 5 (20) | PhCH3 | 110 | 17 | 100 | — | — |
| 3c | 4 (20) | PhCH3 | 110 | 20 | 63 | 37 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 4d | 4 (20) | PhCH3 | 150 | 1 | 50 | 50(80)e | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 5 | 4 (20) | PhCH3 | 110 | 4 | 93 | 7 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 6 | 4 (20) | PhCH3 | 120 | 2 | 80 | 20 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 7 | 4 (10) | PhCH3 | 120 | 1 | 90 | 10 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 8 | 4 (20)f | PhCH3 | 175 | 2 | 53g | 37 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 9 | 7 (20) | PhCH3 | 150 | 1 | 54g | 18 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 10 | 7 (20) | DCB | 200 | 1 | 60g | 30 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 11h | 7 (20) | DCB | 200 | 2 | 20 | 5 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
To select the most suitable catalyst and reaction conditions, initial investigations were carried out under thermal heating using catalysts 4 and 5 (entries 1–3). Reactions in refluxing CH2Cl2 with 4 or toluene with 5 failed to generate any CM product (entries 1 and 2). Fortunately, even though the reaction did not reach complete conversion after 20 h in refluxing toluene, the coupled product was obtained in 37% yield and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Z/E ratio (entry 3) using 4. With the above results in hand, we carried out additional experiments under MW irradiation to shorten the prolonged reaction time and elevated temperature necessary for these CM reactions, which usually leads to thermal degradation of the ruthenium catalysts.21 Despite its widespread application in catalytic reactions, microwave-assisted metathesis reactions have only recently gained increasing popularity.22 In particular, recent reports of dramatic improvements in reaction rates and yields in challenging CM reactions provided by MW irradiation23 prompted us to explore this avenue (entries 4–11). MW irradiation with readily available Ru-catalyst 4 improved the yield (up to 50%) while impressively reducing the reaction time from 20 to 1 h. Since prolonged reaction times did not increased the conversion, we found that the crude can be purified and subjected to another round of metathesis to finally increase the isolated yield up to 80% (entry 4). Indeed, results from entries 4–6 suggest that the reaction temperature (110–150 °C) reached in the reaction vessel is the more determinant factor in microwave-assisted CM reactions between 2 and phenyl vinyl sulfide.24 Because no decomposition of starting material was observed in any case, the formation of 3 seems to be only dependent on the catalytically active ruthenium species, which is also reflected in the correlation of yield with the relative amount of the employed ruthenium complex 4 (entries 6 and 7). However, it is worth noting that a reaction temperature higher than 150 °C did not always lead to a higher conversion. Thus, raising the temperature to 175 °C resulted in a decreased yield (37%), most likely due to catalyst decomposition as well as to the formation of 10% of isomerized byproduct 6. In addition, no significant improvement in the conversion was observed when catalyst 4 was added in two portions separated by a 1 h period (entry 8). We next investigated the reactivity of Hoveyda–Grubbs catalyst 7. The reaction in toluene afforded 18% conversion to CM product 3 and 10% to isomerized 6 (entry 9). Interestingly, by changing the solvent from toluene to 1,2-dichlorobenzene (DCB) and increasing the temperature from 150 to 200 °C, the conversion increased from 18 to 30%. Furthermore, this change in the solvent properties resulted in a reduction of 6 from 28 to 10% (entry 10). Repeating the reaction using 2,6-dichloro-1,4-benzoquinone (10 mol%) as an additive to prevent olefin isomerization25,26 led only to the CM product in 5% conversion and 1
1 Z/E ratio (entry 3) using 4. With the above results in hand, we carried out additional experiments under MW irradiation to shorten the prolonged reaction time and elevated temperature necessary for these CM reactions, which usually leads to thermal degradation of the ruthenium catalysts.21 Despite its widespread application in catalytic reactions, microwave-assisted metathesis reactions have only recently gained increasing popularity.22 In particular, recent reports of dramatic improvements in reaction rates and yields in challenging CM reactions provided by MW irradiation23 prompted us to explore this avenue (entries 4–11). MW irradiation with readily available Ru-catalyst 4 improved the yield (up to 50%) while impressively reducing the reaction time from 20 to 1 h. Since prolonged reaction times did not increased the conversion, we found that the crude can be purified and subjected to another round of metathesis to finally increase the isolated yield up to 80% (entry 4). Indeed, results from entries 4–6 suggest that the reaction temperature (110–150 °C) reached in the reaction vessel is the more determinant factor in microwave-assisted CM reactions between 2 and phenyl vinyl sulfide.24 Because no decomposition of starting material was observed in any case, the formation of 3 seems to be only dependent on the catalytically active ruthenium species, which is also reflected in the correlation of yield with the relative amount of the employed ruthenium complex 4 (entries 6 and 7). However, it is worth noting that a reaction temperature higher than 150 °C did not always lead to a higher conversion. Thus, raising the temperature to 175 °C resulted in a decreased yield (37%), most likely due to catalyst decomposition as well as to the formation of 10% of isomerized byproduct 6. In addition, no significant improvement in the conversion was observed when catalyst 4 was added in two portions separated by a 1 h period (entry 8). We next investigated the reactivity of Hoveyda–Grubbs catalyst 7. The reaction in toluene afforded 18% conversion to CM product 3 and 10% to isomerized 6 (entry 9). Interestingly, by changing the solvent from toluene to 1,2-dichlorobenzene (DCB) and increasing the temperature from 150 to 200 °C, the conversion increased from 18 to 30%. Furthermore, this change in the solvent properties resulted in a reduction of 6 from 28 to 10% (entry 10). Repeating the reaction using 2,6-dichloro-1,4-benzoquinone (10 mol%) as an additive to prevent olefin isomerization25,26 led only to the CM product in 5% conversion and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 Z/E ratio, although this may be a reflection of incompatibility of either 2 or phenyl vinyl sulfide with the additive (entry 11).
2 Z/E ratio, although this may be a reflection of incompatibility of either 2 or phenyl vinyl sulfide with the additive (entry 11).
Collectively, the above observations suggest that the formation of Fischer-type carbenes during the Ru-catalyzed CM reactions between electron-rich phenyl vinyl sulfide and terminal 2-deoxysugar hydroxyalkene 2 decreases productive CM drastically, although good conversions (up to 80%) are still achieved using a 40 mol% of catalyst loading. The higher concentration of reactive phenyl vinyl sulfide olefin causes less interaction between the catalyst and the hydroxyalkene, reducing the formation of active vinylalkylidene species and necessitating higher temperatures in order to achieve significant conversion. Importantly, this elevated concentrations are otherwise required since the stepwise addition of increasing amounts of phenyl vinyl sulfide showed the incipient detection of small amounts of undesired dimerization byproducts.
The substrate scope was expanded next using alkenols 8a–c with phenyl vinyl sulfide as electron-rich olefin partner under optimized reaction conditions (Scheme 2). Despite the expected negative outcome observed with allyl alcohol, due to the known isomerization process previously shown in this type of compounds and their ether counterparts with both first and second Grubbs catalysts,27 cross-metathesis products 9b,c where pleasingly obtained in good yields (up to 66% after three reaction cycles) and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Z/E ratio.
1 Z/E ratio.
 | ||
Scheme 2 Reaction scope. a The starting material decomposed. b Isolated yield after three consecutive reaction cycles (60 mol% of catalyst loading in total) and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Z/E ratio. 1 Z/E ratio. | ||
Additional experiments with phenyl vinyl selenide as electron-rich olefin partner to afford selanyl alkenes28 with the ultimate goal of developing the corresponding selenoglycosyl donors proved unsuccessful.
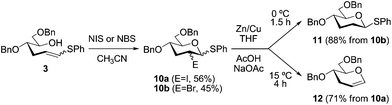
Having established a flexible method for accessing workable amounts of acyclic hexosulfanyl alkene 3, we next demonstrated that this intermediate is able to generate a hexopyranose ring system, in a single step, upon 6-endo electrophilic cyclization29 (Scheme 3). Thus, NIS- or NBS-induced 6-endo cyclization afforded intermediates 10a,b in moderate yields (up to 56%) and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 epimeric mixtures at both C-1 and C-2 (for 10a) that were subsequently transformed into 1-thioglycosyl donor 11 (88% from 10b) and 3-deoxyglycal 12 (71% from 10a) after C–halogen reduction.30 These results reinforce the strategic character of 3 since these privileged D-hexopyranose building blocks are obtained from a single precursor and, for example, they can be used for the stereoselective synthesis of naturally occurring 2,3,6-trideoxy (D-amicetose)-containing oligosaccharides1,7 and other challenging structures such as marine ladder toxins.31
1 epimeric mixtures at both C-1 and C-2 (for 10a) that were subsequently transformed into 1-thioglycosyl donor 11 (88% from 10b) and 3-deoxyglycal 12 (71% from 10a) after C–halogen reduction.30 These results reinforce the strategic character of 3 since these privileged D-hexopyranose building blocks are obtained from a single precursor and, for example, they can be used for the stereoselective synthesis of naturally occurring 2,3,6-trideoxy (D-amicetose)-containing oligosaccharides1,7 and other challenging structures such as marine ladder toxins.31
Conclusions
In summary, we have demonstrated that the particularly challenging CM of a 2-deoxysugar hydroxyl alkene with electron-rich phenyl vinyl sulfide using readily available Ru-catalysts as a key step can be applied to the construction of 2,3-dideoxy-D-ribopyranose ring system donors and analogs. MW irradiation allows the required high reaction temperature to be reached quickly and homogeneously, thereby providing enough energy for a successful metathesis reaction.Experimental section
Proton (1H NMR) and carbon (13C NMR) nuclear magnetic resonance spectra were recorded on a (400 MHz for 1H) and (100.6 MHz for 13C) spectrometer. Spectra were fully assigned using COSY, HSQC, HMBC and NOESY. All chemical shifts are quoted on the δ scale in ppm using residual solvent as the internal standard (1H NMR: CDCl3 = 7.26, CD3OD = 4.87; and 13C NMR: CDCl3 = 77.23; CD3OD = 49.0). Coupling constants (J) are reported in Hz with the following splitting abbreviations: s = singlet, d = doublet, t = triplet, q = quartet, quin = quintet and app = apparent. Infrared (IR) spectra were recorded on a Jasco FT/IR-600 Plus ATR Specac Golden Gate spectrophotometer. Absorption maxima (νmax) are reported in wavenumbers (cm−1). Elemental analyses (C, H, N, and S) were performed with a Carlo Erba EA 1108 Analyzer in the Servei de Recursos Científics (URV). Optical rotations were recorded on a Perkin-Elmer 241 MC polarimeter in a 1 dm cell at 20 °C. Concentrations (c) are given in g per 100 mL. Gas chromatography-mass spectrometry (GC-MS) was measured on an Agilent 9575C MSD apparatus with electronic impact ionization (EI, 70 eV). High resolution mass spectra (HRMS) were recorded on a Waters LCT Premier liquid chromatograph coupled time-of-flight mass spectrometer (HPLC-MS-TOF) with either electrospray ionization (ESI) or atmospheric pressure chemical ionization (APCI) by the ICIQ MS unit. Nominal and exact m/z values are reported in Daltons. Thin layer chromatography (TLC) was carried out using commercial aluminium backed sheets coated with 60F254 silica gel. Visualization of the silica plates was achieved using a UV lamp (λmax = 254 nm) and/or 6% H2SO4 in EtOH and/or 2% PdCl2 and 15% H2SO4 in water. Flash column chromatography was carried out using silica gel 60 (40–63 μm). Radial chromatography was performed on 1, 2, or 4 mm plates of Kieselgel 60 PF254 silica gel, depending on the amount of product. Mobile phases are reported in relative composition (e.g. 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 EtOAc–hexane v/v). All other reagents and anhydrous solvents (Analytical or HPLC grade) were used as received from commercial suppliers. All reactions using anhydrous conditions were performed using flame-dried apparatus under an atmosphere of argon.
1 EtOAc–hexane v/v). All other reagents and anhydrous solvents (Analytical or HPLC grade) were used as received from commercial suppliers. All reactions using anhydrous conditions were performed using flame-dried apparatus under an atmosphere of argon.
(Z/E)-4,6-Di-O-benzyl-1,2,3-trideoxy-1-phenylsulfanyl-D-erythro-hex-1-enitol (3)
A solution of 2 (ref. 20) (40 mg, 0.13 mmol), phenyl vinyl sulfide (86 μL, 0.64 mmol) and catalyst 4 (22 mg, 20 mol%) in dry and degassed toluene (256 μL) was microwave irradiated in a sealed tube at 150 °C for 1 h (temperature control using an external surface sensor, fixed hold time off, normal absorption mode) using a CEM-Discover™ single-mode synthesizer. The residue was filtered through a short path of silica (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 EtOAc–petrol) and the solvent evaporated. The crude was subsequently subjected to a second round of the initial reaction conditions (two reaction cycles in total). The solvent was then evaporated and the crude product was purified by radial chromatography (from hexane to 1
1 EtOAc–petrol) and the solvent evaporated. The crude was subsequently subjected to a second round of the initial reaction conditions (two reaction cycles in total). The solvent was then evaporated and the crude product was purified by radial chromatography (from hexane to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 EtOAc–hexane) to afford 3 (32 mg, 80%) as an inseparable 1
3 EtOAc–hexane) to afford 3 (32 mg, 80%) as an inseparable 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Z/E mixture as a colourless syrup. Rf (1
1 Z/E mixture as a colourless syrup. Rf (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 EtOAc–hexane): 0.28. Data for 3E: 1H NMR (400 MHz, CDCl3) δ 7.36–7.25 (m, 15H), 6.24 (d, J = 15.0 Hz, 1H), 6.00 (ddd, J = 15.0, 7.5 and 7.5 Hz, 1H), 4.67–4.49 (m, 4H), 3.87–3.84 (m, 1H), 3.70–3.53 (m, 3H), 2.66–2.64 (m, 1H), 2.54–2.43 (m, 1H), 2.44 (d, J = 5.2 Hz, 1H); 13C NMR (100.6 MHz, CDCl3) δ 138.4–124.5, 128.6, 125.4, 79.0, 73.6, 72.3, 71.5, 71.2, 34.1. Data for 3Z: 1H NMR (400 MHz, CDCl3) δ 7.36–7.25 (m, 15H), 6.31 (d, J = 11.0 Hz, 1H), 5.94 (ddd, J = 11.0, 7.2 and 7.2 Hz, 1H), 4.67–4.49 (m, 4H), 3.89–3.86 (m, 1H), 3.70–3.53 (m, 3H), 2.66–2.64 (m, 1H), 2.54–2.43 (m, 1H), 2.49 (d, J = 4.8 Hz, 1H); 13C NMR (100.6 MHz, CDCl3) δ 138.3–124.2, 128.5, 125.4, 78.8, 73.6, 72.5, 71.7, 71.1, 30.0. Spectroscopic data are consistent with those reported.29
4 EtOAc–hexane): 0.28. Data for 3E: 1H NMR (400 MHz, CDCl3) δ 7.36–7.25 (m, 15H), 6.24 (d, J = 15.0 Hz, 1H), 6.00 (ddd, J = 15.0, 7.5 and 7.5 Hz, 1H), 4.67–4.49 (m, 4H), 3.87–3.84 (m, 1H), 3.70–3.53 (m, 3H), 2.66–2.64 (m, 1H), 2.54–2.43 (m, 1H), 2.44 (d, J = 5.2 Hz, 1H); 13C NMR (100.6 MHz, CDCl3) δ 138.4–124.5, 128.6, 125.4, 79.0, 73.6, 72.3, 71.5, 71.2, 34.1. Data for 3Z: 1H NMR (400 MHz, CDCl3) δ 7.36–7.25 (m, 15H), 6.31 (d, J = 11.0 Hz, 1H), 5.94 (ddd, J = 11.0, 7.2 and 7.2 Hz, 1H), 4.67–4.49 (m, 4H), 3.89–3.86 (m, 1H), 3.70–3.53 (m, 3H), 2.66–2.64 (m, 1H), 2.54–2.43 (m, 1H), 2.49 (d, J = 4.8 Hz, 1H); 13C NMR (100.6 MHz, CDCl3) δ 138.3–124.2, 128.5, 125.4, 78.8, 73.6, 72.5, 71.7, 71.1, 30.0. Spectroscopic data are consistent with those reported.29
(Z/E)-4-Phenylsulfanyl-3-buten-1-ol (9b)
A solution of 3-buten-1-ol 8b (17 mg, 0.23 mmol), phenyl vinyl sulfide (156 μL, 1.15 mmol) and catalyst 4 (39 mg, 20 mol%) in dry and degassed toluene (462 μL) was microwave irradiated in a sealed tube at 150 °C for 1 h (temperature control using an external surface sensor, fixed hold time off, normal absorption mode) using a CEM-Discover™ single-mode synthesizer. The residue was filtered through a short path of silica (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 EtOAc–petrol) and the solvent evaporated. The crude was subsequently subjected to a second round of the initial reaction conditions (this protocol was repeated up to three reaction cycles in total). The solvent was then evaporated and the crude product was purified by column chromatography (1
1 EtOAc–petrol) and the solvent evaporated. The crude was subsequently subjected to a second round of the initial reaction conditions (this protocol was repeated up to three reaction cycles in total). The solvent was then evaporated and the crude product was purified by column chromatography (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 EtOAc–hexane) to afford 9b (25 mg, 60%) as an inseparable 1
3 EtOAc–hexane) to afford 9b (25 mg, 60%) as an inseparable 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Z/E mixture as a brownish syrup. Rf (1
1 Z/E mixture as a brownish syrup. Rf (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 EtOAc–hexane): 0.17; IR (ATR) νmax/cm−1 3345, 3259, 3056, 2923, 2853, 1749, 1583, 1479, 1439, 1240, 1047, 741; MS (EI, 70 eV) m/z (%) 180.1 (52) [M]+, 149.1 (100), 134.1 (35), 116.1 (61), 109.1 (13), 103.1 (3), 91.1 (4), 85.0 (4), 77.1 (12), 71.0 (6), 65.1 (9), 51.0 (10); HRMS (APCI) m/z calcd for C10H13OS [M + H]+ 181.0682, found 181.0683. Data for 9bE: 1H NMR (400 MHz, CDCl3) δ 7.38–7.17 (m, 5H), 6.29 (dd, J = 15.2 and 1.6 Hz, 1H), 5.92 (dt, J = 15.2 and 7.4 Hz, 1H), 3.72 (bt, J = 6.2 Hz, 2H), 2.44 (ddd, J = 13.6, 6.2 and 1.6 Hz, 2H), 1.46 (bs, 1H); 13C NMR (100.6 MHz, CDCl3) δ 136.1–126.7, 125.0, 62.0, 32.9. Data for 9bZ: 1H NMR (400 MHz, CDCl3) δ 7.38–7.17 (m, 5H), 6.37 (dd, J = 9.3 and 1.6 Hz, 1H), 5.86 (dt, J = 9.3 and 7.4, 1H), 3.77 (bt, J = 6.2 Hz, 2H), 2.55 (ddd, J = 14.0, 6.2 and 1.6 Hz, 2H), 1.46 (bs, 1H); 13C NMR (100.6 MHz, CDCl3) δ 136.1–126.7, 126.3, 62.1, 36.6. Spectroscopic data are consistent with those reported.32
3 EtOAc–hexane): 0.17; IR (ATR) νmax/cm−1 3345, 3259, 3056, 2923, 2853, 1749, 1583, 1479, 1439, 1240, 1047, 741; MS (EI, 70 eV) m/z (%) 180.1 (52) [M]+, 149.1 (100), 134.1 (35), 116.1 (61), 109.1 (13), 103.1 (3), 91.1 (4), 85.0 (4), 77.1 (12), 71.0 (6), 65.1 (9), 51.0 (10); HRMS (APCI) m/z calcd for C10H13OS [M + H]+ 181.0682, found 181.0683. Data for 9bE: 1H NMR (400 MHz, CDCl3) δ 7.38–7.17 (m, 5H), 6.29 (dd, J = 15.2 and 1.6 Hz, 1H), 5.92 (dt, J = 15.2 and 7.4 Hz, 1H), 3.72 (bt, J = 6.2 Hz, 2H), 2.44 (ddd, J = 13.6, 6.2 and 1.6 Hz, 2H), 1.46 (bs, 1H); 13C NMR (100.6 MHz, CDCl3) δ 136.1–126.7, 125.0, 62.0, 32.9. Data for 9bZ: 1H NMR (400 MHz, CDCl3) δ 7.38–7.17 (m, 5H), 6.37 (dd, J = 9.3 and 1.6 Hz, 1H), 5.86 (dt, J = 9.3 and 7.4, 1H), 3.77 (bt, J = 6.2 Hz, 2H), 2.55 (ddd, J = 14.0, 6.2 and 1.6 Hz, 2H), 1.46 (bs, 1H); 13C NMR (100.6 MHz, CDCl3) δ 136.1–126.7, 126.3, 62.1, 36.6. Spectroscopic data are consistent with those reported.32
(Z/E)-10-Phenylsulfanyl-9-decen-1-ol (9c)
A solution of 9-decen-1-ol 9c (37.2 mg, 0.23 mmol), phenyl vinyl sulfide (156 μL, 1.15 mmol) and catalyst 4 (39 mg, 20 mol%) in dry and degassed toluene (462 μL) was microwave irradiated in a sealed tube at 150 °C for 1 h (temperature control using an external surface sensor, fixed hold time off, normal absorption mode) using a CEM-Discover™ single-mode synthesizer. The residue was filtered through a short path of silica (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 EtOAc–petrol) and the solvent evaporated. The crude was subsequently subjected to a second round of the initial reaction conditions (this protocol was repeated up to three reaction cycles in total). The solvent was then evaporated and the crude product was purified by column chromatography (1
1 EtOAc–petrol) and the solvent evaporated. The crude was subsequently subjected to a second round of the initial reaction conditions (this protocol was repeated up to three reaction cycles in total). The solvent was then evaporated and the crude product was purified by column chromatography (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 EtOAc–hexane) to afford 9c (40.2 mg, 66%) as an inseparable 1
3 EtOAc–hexane) to afford 9c (40.2 mg, 66%) as an inseparable 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Z/E mixture as a brownish syrup. Rf (1
1 Z/E mixture as a brownish syrup. Rf (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 EtOAc–hexane): 0.28; IR (ATR) νmax/cm−1 3364, 3074, 2925, 2853, 1709, 1584, 1479, 1439, 1361, 1220, 1054, 949, 909, 737; HRMS (ESI) m/z calcd for C16H24NaOS [M + Na]+ 287.1414, found 287.1435. Data for 9cE: 1H NMR (400 MHz, CDCl3) δ 7.36–7.17 (m, 5H), 6.14 (dd, J = 14.8 and 1.2 Hz, 1H), 6.01 (dt, J = 14.8 and 7.0 Hz, 1H), 3.69–3.62 (m, 2H), 2.17 (ddd, J = 14.4, 7.0 and 1.2 Hz, 2H), 1.60–1.23 (m, 12H); 13C NMR (100.6 MHz, CDCl3) δ 137.9–126.2, 120.9, 63.3, 33.3–25.9. Data for 9cZ: 1H NMR (400 MHz, CDCl3) δ 7.36–7.17 (m, 5H), 6.20 (dd, J = 9.3 and 1.6 Hz, 1H), 5.83 (dt, J = 9.3 and 7.4, 1H), 3.69–3.62 (m, 2H), 2.26 (ddd, J = 14.4, 7.4 and 1.6 Hz, 2H), 1.60–1.23 (m, 12H); 13C NMR (100.6 MHz, CDCl3) δ 136.8–126.2, 122.8, 63.3, 33.3–25.9.
3 EtOAc–hexane): 0.28; IR (ATR) νmax/cm−1 3364, 3074, 2925, 2853, 1709, 1584, 1479, 1439, 1361, 1220, 1054, 949, 909, 737; HRMS (ESI) m/z calcd for C16H24NaOS [M + Na]+ 287.1414, found 287.1435. Data for 9cE: 1H NMR (400 MHz, CDCl3) δ 7.36–7.17 (m, 5H), 6.14 (dd, J = 14.8 and 1.2 Hz, 1H), 6.01 (dt, J = 14.8 and 7.0 Hz, 1H), 3.69–3.62 (m, 2H), 2.17 (ddd, J = 14.4, 7.0 and 1.2 Hz, 2H), 1.60–1.23 (m, 12H); 13C NMR (100.6 MHz, CDCl3) δ 137.9–126.2, 120.9, 63.3, 33.3–25.9. Data for 9cZ: 1H NMR (400 MHz, CDCl3) δ 7.36–7.17 (m, 5H), 6.20 (dd, J = 9.3 and 1.6 Hz, 1H), 5.83 (dt, J = 9.3 and 7.4, 1H), 3.69–3.62 (m, 2H), 2.26 (ddd, J = 14.4, 7.4 and 1.6 Hz, 2H), 1.60–1.23 (m, 12H); 13C NMR (100.6 MHz, CDCl3) δ 136.8–126.2, 122.8, 63.3, 33.3–25.9.
Phenyl 4,6-di-O-benzyl-2,3-dideoxy-2-iodo-1-thio-α/β-D-arabino/ribo-hexopyranoside (10a)
NIS (164.1 mg, 0.67 mmol) was added to a solution of 3 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Z/E) (180 mg, 0.43 mmol) in dry CH3CN (3.5 mL) at −30 °C and stirred for 0.5 h. The mixture was diluted with CH2Cl2 and washed with saturated aqueous Na2S2O3. The combined organic layers were dried over MgSO4, filtered and concentrated. The residue was purified by radial chromatography (from hexane to 1
1 Z/E) (180 mg, 0.43 mmol) in dry CH3CN (3.5 mL) at −30 °C and stirred for 0.5 h. The mixture was diluted with CH2Cl2 and washed with saturated aqueous Na2S2O3. The combined organic layers were dried over MgSO4, filtered and concentrated. The residue was purified by radial chromatography (from hexane to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 EtOAc–hexane) to afford 10a (130 mg, 56%) as a 1
3 EtOAc–hexane) to afford 10a (130 mg, 56%) as a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 α/β mixture and 1
1 α/β mixture and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ax/eq. mixture at C-2 (arabino/ribo) as a yellowish syrup. Selected data for ribo-10a: 1H NMR (400 MHz, CDCl3) δ 7.36–7.19 (m, 15H), 4.83 (dd, J = 9.9 and 5.1 Hz, 1H), 4.82 (dd, J = 10.0 and 4.4 Hz, 1H), 4.22 (m, 1H), 3.86 (ddd, J = 13.0, 9.5 and 4.4 Hz, 1H), 3.69 (m, 2H), 3.66 (m, 2H), 3.49 (dt, J = 10.8 and 4.8 Hz, 1H), 3.45 (ddd, J = 10.4, 9.5 and 4.4 Hz, 1H), 2.85 (dt, J = 12.8 and 4.4 Hz, 1H), 2.60 (dt, J = 12.2 and 4.7 Hz, 1H), 2.45 (dt, J = 13.2 and 9.8 Hz, 1H), 2.10 (ddd, J = 13.0, 12.8 and 10 Hz, 1H); 13C NMR (100.6 MHz, CDCl3) δ 138.4–124.2, 99.2, 92.1, 79.0–53.6, 41.7, 35.9, 25.7, 22.6. Selected data for arabino-10a: 1H NMR (400 MHz, CDCl3) δ 7.36–7.18 (m, 15H), 4.43 (s, 1H), 4.27 (s, 1H), 4.16 (m, 1H), 3.90 (m, 1H), 3.80 (m, 2H), 3.73 (m, 2H), 3.67 (m, 2H), 2.60 (ddd, J = 14.5, 4.2 and 3.7 Hz, 1H), 2.30–2.17 (m, 2H), 2.05 (ddd, J = 14.5, 10.2 and 3.6 Hz, 1H); 13C NMR (100.6 MHz, CDCl3) δ 138.3–127.7, 93.4, 84.6, 79.2–69.5, 37.0, 33.1, 29.6, 26.8.
1 ax/eq. mixture at C-2 (arabino/ribo) as a yellowish syrup. Selected data for ribo-10a: 1H NMR (400 MHz, CDCl3) δ 7.36–7.19 (m, 15H), 4.83 (dd, J = 9.9 and 5.1 Hz, 1H), 4.82 (dd, J = 10.0 and 4.4 Hz, 1H), 4.22 (m, 1H), 3.86 (ddd, J = 13.0, 9.5 and 4.4 Hz, 1H), 3.69 (m, 2H), 3.66 (m, 2H), 3.49 (dt, J = 10.8 and 4.8 Hz, 1H), 3.45 (ddd, J = 10.4, 9.5 and 4.4 Hz, 1H), 2.85 (dt, J = 12.8 and 4.4 Hz, 1H), 2.60 (dt, J = 12.2 and 4.7 Hz, 1H), 2.45 (dt, J = 13.2 and 9.8 Hz, 1H), 2.10 (ddd, J = 13.0, 12.8 and 10 Hz, 1H); 13C NMR (100.6 MHz, CDCl3) δ 138.4–124.2, 99.2, 92.1, 79.0–53.6, 41.7, 35.9, 25.7, 22.6. Selected data for arabino-10a: 1H NMR (400 MHz, CDCl3) δ 7.36–7.18 (m, 15H), 4.43 (s, 1H), 4.27 (s, 1H), 4.16 (m, 1H), 3.90 (m, 1H), 3.80 (m, 2H), 3.73 (m, 2H), 3.67 (m, 2H), 2.60 (ddd, J = 14.5, 4.2 and 3.7 Hz, 1H), 2.30–2.17 (m, 2H), 2.05 (ddd, J = 14.5, 10.2 and 3.6 Hz, 1H); 13C NMR (100.6 MHz, CDCl3) δ 138.3–127.7, 93.4, 84.6, 79.2–69.5, 37.0, 33.1, 29.6, 26.8.
Phenyl 4,6-di-O-benzyl-2,3-dideoxy-1-thio-α/β-D-erythro-hexopyranoside (11)
NBS (63.5 mg, 0.36 mmol) was added to a solution of 3 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Z/E) (100 mg, 0.23 mmol) in dry CH3CN (3.4 mL) at −30 °C and stirred for 2.5 h. The mixture was diluted with CH2Cl2 and washed with saturated aqueous Na2S2O3. The combined organic layers were dried over MgSO4, filtered and concentrated. The residue was filtered through a short silica plug (from hexane to 1
1 Z/E) (100 mg, 0.23 mmol) in dry CH3CN (3.4 mL) at −30 °C and stirred for 2.5 h. The mixture was diluted with CH2Cl2 and washed with saturated aqueous Na2S2O3. The combined organic layers were dried over MgSO4, filtered and concentrated. The residue was filtered through a short silica plug (from hexane to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 EtOAc–hexane) and the solvent evaporated to afford phenyl 4,6-di-O-benzyl-2,3-dideoxy-2-bromo-1-thio-α/β-D-arabino/ribo-hexopyranoside 10b (53 mg, 45%) as a brownish syrup. The isolated product decomposed on standing and was therefore quickly subjected to the next reaction. A mixture of 10b (40 mg, 0.08 mmol) and NaOAc (9.6 mg, 0.12 mmol) were dissolved in THF (0.5 mL) and acetic acid (7 μL) at 0 °C. Zn/Cu couple (53 mg) was then added and the reaction was left to stir at the same temperature for 1.5 h. The mixture was then diluted with CH2Cl2 and washed with saturated aqueous NaHCO3. The combined organic layers were dried over MgSO4, filtered and concentrated. The residue was purified by radial chromatography (from hexane to 1
3 EtOAc–hexane) and the solvent evaporated to afford phenyl 4,6-di-O-benzyl-2,3-dideoxy-2-bromo-1-thio-α/β-D-arabino/ribo-hexopyranoside 10b (53 mg, 45%) as a brownish syrup. The isolated product decomposed on standing and was therefore quickly subjected to the next reaction. A mixture of 10b (40 mg, 0.08 mmol) and NaOAc (9.6 mg, 0.12 mmol) were dissolved in THF (0.5 mL) and acetic acid (7 μL) at 0 °C. Zn/Cu couple (53 mg) was then added and the reaction was left to stir at the same temperature for 1.5 h. The mixture was then diluted with CH2Cl2 and washed with saturated aqueous NaHCO3. The combined organic layers were dried over MgSO4, filtered and concentrated. The residue was purified by radial chromatography (from hexane to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 EtOAc–hexane) to afford 11 (31 mg, 88%) as a colourless syrup. 1H NMR (400 MHz, CDCl3) δ 7.61–7.22 (m, 15H), 4.71 (d, J = 10.4 Hz, 1H), 4.61–4.40 (m, 4H), 3.80–3.68 (m, 4H), 3.58 (ddd, J = 9.6, 4.8 and 2.0 Hz, 1H), 3.47 (ddd, J = 9.6, 10.4 and 4.4 Hz, 1H), 2.88 (ddd, J = 12.4, 4.8 and 4.4 Hz, 1H), 2.03 (ddd, J = 12.4, 11.2 and 10.4 Hz, 1H); 13C NMR (100.6 MHz, CDCl3) δ 133.4–127.8, 89.5, 82.0, 73.6, 72.7, 71.7, 69.3, 45.7, 41.9. Spectroscopic data are consistent with those reported.30
5 EtOAc–hexane) to afford 11 (31 mg, 88%) as a colourless syrup. 1H NMR (400 MHz, CDCl3) δ 7.61–7.22 (m, 15H), 4.71 (d, J = 10.4 Hz, 1H), 4.61–4.40 (m, 4H), 3.80–3.68 (m, 4H), 3.58 (ddd, J = 9.6, 4.8 and 2.0 Hz, 1H), 3.47 (ddd, J = 9.6, 10.4 and 4.4 Hz, 1H), 2.88 (ddd, J = 12.4, 4.8 and 4.4 Hz, 1H), 2.03 (ddd, J = 12.4, 11.2 and 10.4 Hz, 1H); 13C NMR (100.6 MHz, CDCl3) δ 133.4–127.8, 89.5, 82.0, 73.6, 72.7, 71.7, 69.3, 45.7, 41.9. Spectroscopic data are consistent with those reported.30
1,5-Anhydro-4,6-di-O-benzyl-D-erythro-hex-1-enitol (12)
A mixture of 10a (130 mg, 0.24 mmol) and NaOAc (27 mg, 0.33 mmol) were dissolved in THF (0.5 mL) and acetic acid (20 μL) at 0 °C. Zn/Cu couple (160 mg) was then added and the reaction mixture was warmed to 15 °C and stirred for 4 h. The mixture was then diluted with CH2Cl2 and washed with saturated aqueous NaHCO3. The combined organic layers were dried over MgSO4, filtered and concentrated. The residue was purified by radial chromatography (from hexane to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 EtOAc–hexane) to afford 12 (50 mg, 71%) as a colourless syrup. Rf (1
4 EtOAc–hexane) to afford 12 (50 mg, 71%) as a colourless syrup. Rf (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 EtOAc–hexane): 0.40; 1H NMR (400 MHz, CDCl3) δ 7.36–7.21 (m, 10H), 6.36 (ddd, J = 6.4, 2.4 and 1.6 Hz, 1H), 4.63 (ddd, J = 6.4, 5.2 and 2.6 Hz, 1H), 4.62–4.50 (m, 4H), 3.90 (dd, J = 8.0 and 4.0 Hz, 1H), 3.79 (m, 3H), 2.38 (dddd, J = 16.4, 6.0, 5.2 and 1.6 Hz, 1H), 2.08 (dddd, J = 16.4, 8.4, 2.6 and 2.4 Hz, 1H); 13C NMR (100.6 MHz, CDCl3) δ 143.3, 138.4–127.8, 97.8, 76.9, 73.7, 71.3, 70.7, 69.2, 26.7. Spectroscopic data are consistent with those reported.30
3 EtOAc–hexane): 0.40; 1H NMR (400 MHz, CDCl3) δ 7.36–7.21 (m, 10H), 6.36 (ddd, J = 6.4, 2.4 and 1.6 Hz, 1H), 4.63 (ddd, J = 6.4, 5.2 and 2.6 Hz, 1H), 4.62–4.50 (m, 4H), 3.90 (dd, J = 8.0 and 4.0 Hz, 1H), 3.79 (m, 3H), 2.38 (dddd, J = 16.4, 6.0, 5.2 and 1.6 Hz, 1H), 2.08 (dddd, J = 16.4, 8.4, 2.6 and 2.4 Hz, 1H); 13C NMR (100.6 MHz, CDCl3) δ 143.3, 138.4–127.8, 97.8, 76.9, 73.7, 71.3, 70.7, 69.2, 26.7. Spectroscopic data are consistent with those reported.30
Acknowledgements
We thank the Ministerio de Economía y Competitividad, Spain (CTQ2011-22872BQU) for generous financial support. We also thank Dr I. Marín for preliminary results and Ms. M. Salvadó, Ms. E. Bresó, Dr A. Lishchynskyi and the ICIQ MS unit for technical assistance. O.B. thanks the Ministerio de Ciencia e Innovación, Spain (Juan de la Cierva Fellowship) and the European Commission (Marie Curie Career Integration Grant).Notes and references
- R. M. de Lederkremer and C. Marino, in Adv. Carbohydr. Chem. Biochem., ed. D. Horton, Elsevier, Amsterdam, 2007, vol. 61, pp. 143–216 Search PubMed.
- S. Kunimoto, J. Lu, H. Esumi, Y. Yamazaki, N. Kinoshita, Y. Honma, M. Hamada, M. Ohsono, M. Ishizuka and T. Takeuchi, J. Antibiot., 2003, 56, 1004 CrossRef CAS.
- B. Ostash, A. Korynevska, R. Stoika and V. Fedorenko, Mini-Rev. Med. Chem., 2009, 9, 1040 CrossRef CAS.
- A. Luzhetskyy, A. Vente and A. Bechthold, Mol. BioSyst., 2005, 1, 117 RSC.
- C. L. Stevens, K. Nagarajan and T. H. Haskell, J. Org. Chem., 1962, 27, 2991 CrossRef CAS.
- A. Eschenmoser, Angew. Chem., Int. Ed., 2011, 50, 12412 CrossRef CAS PubMed and references therein.
- C. L. Stevens, P. Blumbergs and D. L. Wood, J. Am. Chem. Soc., 1964, 86, 3592 CrossRef CAS.
- (a) R. S. Babu, Q. Chen, S.-W. Kang, M. Zhou and G. A. O'Doherty, J. Am. Chem. Soc., 2012, 134, 11952 CrossRef CAS PubMed and references therein; (b) I. Cobo, M. I. Matheu, S. Castillón, O. Boutureira and B. G. Davis, Org. Lett., 2012, 14, 1728 CrossRef CAS PubMed; (c) M. H. Haukaas and G. A. O'Doherty, Org. Lett., 2002, 4, 1771 CrossRef CAS PubMed.
- A. Aljarilla, J. C. López and J. Plumet, Eur. J. Org. Chem., 2010, 6123 CrossRef CAS.
- (a) C. Samojłowicz and K. Grela, ARKIVOC, 2011, 71 Search PubMed; (b) P. van de Weghe, P. Bisseret, N. Blanchard and J. Eustache, J. Organomet. Chem., 2006, 691, 5078 CrossRef CAS PubMed.
- Y. A. Lin and B. G. Davis, Beilstein J. Org. Chem., 2010, 6, 1219 CrossRef CAS PubMed.
- (a) Y. A. Lin, O. Boutureira, L. Lercher, B. Bhushan, R. S. Paton and B. G. Davis, J. Am. Chem. Soc., 2013, 135, 12156 CrossRef CAS PubMed; (b) Y. A. Lin, J. M. Chalker and B. G. Davis, J. Am. Chem. Soc., 2010, 132, 16805 CrossRef CAS PubMed; (c) J. M. Chalker, Y. A. Lin, O. Boutureira and B. G. Davis, Chem. Commun., 2009, 3714 RSC; (d) Y. A. Lin, J. M. Chalker, M. Floyd, G. J. L. Bernardes and B. G. Davis, J. Am. Chem. Soc., 2008, 130, 9642 CrossRef CAS PubMed.
- S. J. Meek, R. V. O'Brien, J. Llaveria, R. R. Schrock and A. H. Hoveyda, Nature, 2011, 471, 461 CrossRef CAS PubMed.
- (a) J. Carreras, A. Avenoza, J. H. Busto and J. M. Peregrina, J. Org. Chem., 2009, 74, 1736 CrossRef CAS PubMed; (b) Z. Liu and J. D. Rainier, Org. Lett., 2005, 7, 131 CrossRef CAS PubMed and references therein.
- K. F. W. Hekking, F. L. van Delft and F. P. J. T. Rutjes, Tetrahedron, 2003, 59, 6751 CrossRef CAS.
- (a) C. Fischmeister and C. Bruneau, Beilstein J. Org. Chem., 2011, 7, 156 CrossRef CAS PubMed; (b) A. J. Giessert and S. T. Diver, Chem. Rev., 2004, 104, 1317 CrossRef PubMed.
- (a) M. L. Macnaughtan, J. B. Gary, D. L. Gerlach, M. J. A. Johnson and J. W. Kampf, Organometallics, 2009, 28, 2880 CrossRef CAS; (b) V. Sashuk, C. Samojłowicz, A. Szadkowska and K. Grela, Chem. Commun., 2008, 2468 RSC.
- (a) Y. Itami, B. Marciniec and M. Kubicki, Chem.–Eur. J., 2004, 10, 1239 CrossRef CAS PubMed; (b) B. Marciniec, D. Chadyniak and S. Krompiec, J. Mol. Catal. A: Chem., 2004, 224, 111 CrossRef CAS PubMed.
- (a) H. Werner, C. Grünwald, W. Stüer and J. Wolf, Organometallics, 2003, 22, 1558 CrossRef CAS; (b) J. Louie and R. H. Grubbs, Organometallics, 2002, 21, 2153 CrossRef CAS; (c) Z. Wu, S. T. Nguyen, R. H. Grubbs and J. W. Ziller, J. Am. Chem. Soc., 1995, 117, 5503 CrossRef CAS.
- (a) O. R. Ludek and V. E. Marquez, Tetrahedron, 2009, 65, 8461 CrossRef CAS PubMed; (b) N. Hossain, N. Blaton, O. Peeters, J. Rozenski and P. A. Herdewijn, Tetrahedron, 1996, 52, 5563 CrossRef CAS.
- S. H. Hong, M. W. Day and R. H. Grubbs, J. Am. Chem. Soc., 2004, 126, 7414 CrossRef CAS PubMed.
- (a) F. Nicks, Y. Borguet, S. Delfosse, D. Bicchielli, L. Delaude, X. Sauvage and A. Demonceau, Aust. J. Chem., 2009, 62, 184 CrossRef CAS; (b) Y. Coquerel and J. Rodriguez, Eur. J. Org. Chem., 2008, 1125 CrossRef CAS.
- C. Samojłowicz, E. Borré, C. Mauduit and K. Grela, Adv. Synth. Catal., 2011, 353, 1993 CrossRef.
- C. O. Kappe, B. Pieber and D. Dallinger, Angew. Chem., Int. Ed., 2013, 52, 1088 CrossRef CAS PubMed.
- (a) B. Schmidt, J. Mol. Catal. A: Chem., 2006, 254, 53 CrossRef CAS PubMed; (b) B. Schmidt, Eur. J. Org. Chem., 2004, 1865 CrossRef CAS.
- S. H. Hong, D. P. Sanders, C. W. Lee and R. H. Grubbs, J. Am. Chem. Soc., 2005, 127, 17160 CrossRef CAS PubMed.
- B. Schmidt and S. Hauke, Org. Biomol. Chem., 2013, 11, 4194 CAS and references therein.
- O. Boutureira, M. I. Matheu, Y. Díaz and S. Castillón, Carbohydr. Res., 2007, 342, 736 CrossRef CAS PubMed.
- (a) A. Kövér, O. Boutureira, M. I. Matheu, Y. Díaz and S. Castillón, J. Org. Chem., 2014, 79, 3060 CrossRef PubMed; (b) O. Boutureira, M. A. Rodríguez, Y. Díaz, M. I. Matheu and S. Castillón, Carbohydr. Res., 2010, 345, 1041 CrossRef CAS PubMed; (c) O. Boutureira, M. A. Rodríguez, D. Benito, M. I. Matheu, Y. Díaz and S. Castillón, Eur. J. Org. Chem., 2007, 3564 CrossRef CAS; (d) M. A. Rodríguez, O. Boutureira, M. I. Matheu, Y. Díaz and S. Castillón, Eur. J. Org. Chem., 2007, 2470 CrossRef; (e) M. A. Rodríguez, O. Boutureira, M. I. Matheu, Y. Díaz, S. Castillón and P. H. Seeberger, J. Org. Chem., 2007, 72, 8998 CrossRef PubMed; (f) M. A. Rodríguez, O. Boutureira, X. Arnés, Y. Díaz, M. I. Matheu and S. Castillón, J. Org. Chem., 2005, 70, 10297 CrossRef PubMed.
- O. Boutureira, M. A. Rodríguez, M. I. Matheu, Y. Díaz and S. Castillón, Org. Lett., 2006, 8, 673 CrossRef CAS PubMed.
- B. M. Trost and Y. H. Rhee, Org. Lett., 2004, 6, 4311 CrossRef CAS PubMed.
- H. Yorimitsu, K. Wakabayashi, H. Shinokubo and K. Oshima, Bull. Chem. Soc. Jpn., 2001, 74, 1963 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Copies of 1H and 13C NMR spectra for compounds 3, 6, 9c and 11. See DOI: 10.1039/c4ra01668h |
| This journal is © The Royal Society of Chemistry 2014 |