A catalyst-free, facile and efficient approach to cyclic esters: synthesis of 4H-benzo[d][1,3]dioxin-4-ones†
Feng Lin,
Qiuling Song,
Yuyu Gao and
Xiuling Cui*
Engineering Research Center of Molecular Medicine, Ministry of Education, Key Laboratory of Xiamen Marine and Gene Drugs, Institutes of Molecular Medicine and School of Biomedical Sciences, Huaqiao University, Xiamen, 361021, China. E-mail: cuixl@hqu.edu.cn
First published on 6th March 2014
Abstract
We have developed a green and practical method to construct 4H-benzo[d][1,3]dioxin-4-one and its derivatives, which are important structural units in insecticides, and intermediates to synthesize multiple-substituted benzene derivatives of great value. The catalyst- and additive-free conditions, commercial and cheap starting materials and short reaction time, make this transformation practical and attractive.
Introduction
4H-Benzo[d][1,3]dioxin-4-one and its derivatives show great promise for many applications in organic and pharmaceutical synthesis1–23 as well as agriculture.24 However, few methodologies exist for building these structures so far. In a pioneering work, Mowry and co-workers obtained 4H-benzo[d][1,3]dioxin-4-ones from the condensation of salicylic acids with vinyl acetate, catalyzed by mercuric acetate in the presence of sulfuric acid (①, Scheme 1).25,26 To avoid using sulfuric acid or metal magma, more convenient methodologies have been developed. For instance, Perlmutter and co-workers discovered that the reaction of phenyl salicylate with aldehydes could afford the corresponding cyclic products using 1,4-diazabicyclo[2.2.2]octane (DABCO) as a base in 1996 (②, Scheme 1).27 Salicylic acids can be converted into 4H-benzo[d][1,3]-dioxin-4-ones by reacting with a ketone, catalyzed by N,N-4-dimethylamino-pyridine (DMAP) in the presence of stoichiometric SOCl2 (③, Scheme 1)8–19,28,29 and in a solution of trifluoroacetic acid (TFA) and trifluoroacetic anhydride (④, Scheme 1).5,20–22 Instead of TFA and trifluoroacetic anhydride, catalytic amounts of sulfuric acid (H2SO4) and stoichiometric acetic anhydride can be used to prompt this transformation.23 Gandelman and co-workers accidentally produced 4H-benzo[d][1,3]dioxin-4-one via irradiation of a mixture containing o-methylsalicylic acid, 1,3-diiodo-5,5-dimethylhydantoin (DIH) and dichloroethane for 20 h under reflux (⑤, Scheme 1).30However, some challenges still exist in these procedures, such as unavailable starting materials, limitations of substrate scope, unavoidable side reactions and the requirement for strong acids. Therefore, a green, practical and efficient approach for the formation of 4H-benzo[d][1,3]dioxin-4-one and its derivatives from readily available starting materials is extremely desirable. Herein, we present an unprecedented protocol to construct such a structure (⑥, Scheme 1). The significance of this methodology is that: (1) CH2Cl2, a common and cheap reagent in laboratories, has seldom been used as a reagent and C1 source at the same time;31,32 (2) additive and metal free reaction conditions were used; (3) moisture insensitivity and high efficiency were achieved.
Results and discussion
Initially, the reaction of salicylic acid (1a) with CH2Cl2 was chosen as a model reaction to screen the reaction parameters. K3PO4·3H2O was investigated first to screen the reaction temperature and solvent. Only a trace amount of the desired product was observed at 60 °C (Table 1, entry 1). When the temperature was raised to 80 °C, the desired product 2a was achieved in 10% yield (Table 1, entry 2). The yield increased to 99% when the reaction was carried out at 100 °C (Table 1, entry 3). Using DMSO as the solvent also resulted in the same excellent yield (99%) (Table 1, entry 4). However, no product was observed in 1,4-dioxane, toluene and THF (Table 1, entries 5–7). Other bases, such as K2HPO4·3H2O, KHCO3, K2CO3, Na2CO3, NaHCO3, pyridine, Cs2CO3, NaOH, KOH, and NaOEt did not favour this transformation (Table 1, entries 8–17). Without CH2Cl2, the reaction did not proceed (Table 1, entry 18). When carried out in a sealed tube with 0.25 mL of CH2Cl2, the product was observed in 5% yield. When the temperature was increased to 125 °C, the reaction produced the product in 92% yield after 15 h. Similarly, 2a was obtained in 71% yield when the system was charged with 0.1 mL CH2Cl2.| entry | Base | Solvent | t (h) | T (°C) | Yieldb % |
|---|---|---|---|---|---|
| a Reaction conditions: salicylic acid (0.5 mmol), bases (1 mmol), CH2Cl2 (0.6 mL), solvent (1.5 mL).b Isolated yield based on 1a, NR = no reaction.c The reaction was carried out with no CH2Cl2.d CH2Cl2 (0.25 mL), sealed tube.e CH2Cl2 (0.25 mL), sealed tube.f CH2Cl2 (0.1 mL), sealed tube. | |||||
| 1 | K3PO4·3H2O | DMF | 6 | 60 | Trace |
| 2 | K3PO4·3H2O | DMF | 6 | 80 | 10 |
| 3 | K3PO4·3H2O | DMF | 6 | 100 | 99 |
| 4 | K3PO4·3H2O | DMSO | 6 | 100 | 99 |
| 5 | K3PO4·3H2O | 1,4-Dioxane | 6 | 100 | NR |
| 6 | K3PO4·3H2O | Toluene | 6 | 100 | NR |
| 7 | K3PO4·3H2O | THF | 6 | 100 | NR |
| 8 | K2HPO4·3H2O | DMF | 6 | 100 | 15 |
| 9 | KHCO3 | DMF | 6 | 100 | NR |
| 10 | K2CO3 | DMF | 6 | 100 | Trace |
| 11 | Na2CO3 | DMF | 6 | 100 | NR |
| 12 | NaHCO3 | DMF | 6 | 100 | NR |
| 13 | Pyridine | DMF | 6 | 100 | NR |
| 14 | Cs2CO3 | DMF | 6 | 100 | Trace |
| 15 | NaOH | DMF | 6 | 100 | Trace |
| 16 | KOH | DMF | 6 | 100 | Trace |
| 17 | NaOEt | DMF | 6 | 100 | 10 |
| 18c | K3PO4·3H2O | DMF | 6 | 100 | NR |
| 19d | K3PO4·3H2O | DMF | 6 | 100 | <5 |
| 20e | K3PO4·3H2O | DMF | 15 | 125 | 92 |
| 21f | K3PO4·3H2O | DMF | 15 | 125 | 71 |
With the optimal reaction conditions in hand, various salicylic acids 1 were screened. The results are summarized in Table 2. Various groups substituted on the benzene ring, such as methyl, fluoro, chloro, bromo, methoxy, trifluoromethyl, amino and tert-butyl, were tolerated well under the standard reaction conditions and gave excellent yields (Table 2, entries 1–13). Based on this series of experiments, substituents at the ortho-, meta-, and para-positions of the aromatic moiety did not significantly affect the outcome (Table 2, entries 2–13), especially noticing the high reactivity of 1q, with two bulky tert-butyl groups (Table 2, entry 17). Meanwhile, both the electron-rich (Table 2, entries 2–4, entries 11–13, entries 15 and 17) and electron-deficient salicylic acids (Table 2, entries 5–10, entries 14 and 16) gave excellent yields. The trifluoromethyl group, a significant group in the life sciences,33 as well as the unprotected amino group, could be tolerated in this transformation very well (Table 2, entries 14 and 15), affording the desirable products in 75% and 90% yields, respectively. Notably, the halogen groups could survive well under the standard reaction conditions and no cleavage of the C–halogen bond was observed (Table 1, entries 5–10). Salicylic acid with two chlorine groups could also furnish the desired product in 70% yield (Table 2, entry 16). These products with halogen groups could be applied for further functionalization to build useful and more complicated molecules.
| Entry | 1 | Product | Yieldc | Entry | 1 | Product | Yieldc | ||
|---|---|---|---|---|---|---|---|---|---|
| a Reaction conditions: 1 (0.5 mmol), K3PO4·3H2O (1 mmol), CH2Cl2 (0.6 mL), DMF (1.5 mL), 6 h, 100 °C.b Reaction conditions: 1 (0.5 mmol), K3PO4·3H2O (1 mmol), CH3CHCl2 (0.6 mL), DMF (1.5 mL), 10 h, 130 °C.c Isolated yields based on 1.d 6 h.e 8 h. | |||||||||
| 1 |  |
1a | 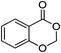 |
2a (>99%) | 14d | 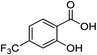 |
1n |  |
2n (75%) |
| 2 |  |
1b |  |
2b (99%) | 15 | 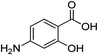 |
1o |  |
2o (90%) |
| 3 | 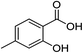 |
1c | 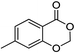 |
2c (99%) | 16e |  |
1p |  |
2p (70%) |
| 4 |  |
1d |  |
2d (98%) | 17 |  |
1q |  |
2q (98%) |
| 5 |  |
1e | 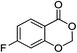 |
2e (97%) | 18 |  |
1a | 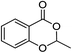 |
3a (65%) |
| 6 | 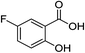 |
1f | 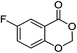 |
2f (89%) | 19 |  |
1b |  |
3b (49%) |
| 7 | 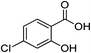 |
1g |  |
2g (95%) | 20 |  |
1c | 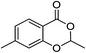 |
3c (53%) |
| 8 |  |
1h |  |
2h (96%) | 21 | 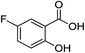 |
1f |  |
3f (55%) |
| 9 |  |
1i | 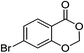 |
2i (90%) | 22 | 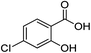 |
1g |  |
3g (43%) |
| 10 | 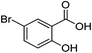 |
1j |  |
2j (92%) | 23 |  |
1k | 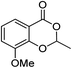 |
3k (43%) |
| 11 |  |
1k | 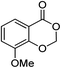 |
2k (98%) | 24 |  |
1m | 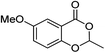 |
3m (51%) |
| 12 | 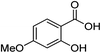 |
1l | 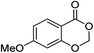 |
2l (99%) | 25 |  |
1q |  |
3q (52%) |
| 13 |  |
1m | 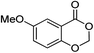 |
2m (95%) | |||||
The above results inspired us to further demonstrate the application of our developed protocol into coupling of salicylic acids with CHCl2CH3 for the synthesis of 2-methyl-substituted 4H-benzo[d][1,3]dioxin-4-ones, as depicted in Table 2 as well as in S3 in ESI,† for screening the reaction conditions. 1a could be smoothly transformed into the desired products in 65% yield (Table 2, entry 18). Either electron-donating groups or electron-withdrawing groups at the aromatic rings of the salicylic acids were well tolerated, such as Me, OMe, F, Cl, (CH3)3C (Table 2, entries 19–25). The steric hindrance of the substituted groups had a slight effect on the transformation. Ortho-substituted salicylic acids were converted to the desired products in relatively lower yields, compared to the ones substituted at the meta- or para-positions (Table 2, entry 19 vs. 20 and entry 23 vs. 24). Substituted salicylic acid 1q with two tert-butyl groups could provide the desired product in 52% yield (Table 2, entry 25). In summary, salicylic acid bearing electron-donating groups (methyl, methoxy) and halogen groups (fluoro, chloro) did not give conspicuous differences in yields, and steric hindrance may have a slight impact on the reaction. The overall yields of CHCl2CH3 are lower than CH2Cl2 as a starting material, which may be due to CHCl2CH3 being more crowded than dichloromethane.
The reaction was successfully performed on a larger scale to demonstrate the practicability of this methodology. Products 2a and 2n could be conveniently obtained on a 15 mmol scale in yields similar to those on a small scale (e.g., 2a: 99% vs. 98% and 2n: 98% vs. 98%) (see S4 and S5 in ESI†).
4H-Benzo[d][1,3]dioxin-4-ones are versatile building blocks in organic synthesis. After this cyclization protocol was established, we looked forward to applying these cyclic products to further transformations (Scheme 2). For the classical hydrolysis reaction, 2a was treated with stoichiometric 48% aqueous KOH, affording the corresponding salicylic acid in 95% yield.22 When 2a was treated with 10 mol% K2CO3 in MeOH, methyl salicylate 1ab was produced in 96% yield. Furthermore, Itaru Sato and co-workers reported that 5 could be successfully converted to 8.21 7-(Bromomethyl)-4H-benzo[d][1,3]dioxin-4-one (5) could be obtained through the reaction of 7-(methyl)-4H-benzo[d][1,3]dioxin-4-one (2c) with N-bromosuccinimide (NBS) in the presence of catalytic amounts of benzoyl peroxide (Bz2O2).5 Upon treatment of 2a with 4 equiv. of LiAlH4, an 82% yield of 2-(hydroxymethyl)phenol (6) was produced.20 2a can also undergo other transformations. For instance, compound 7, a potential ingredient of insecticides, could be prepared using 2a as the starting material,24 thus has the potential to replace its analogues to finish their relative reactions.1–23
Consequently, some controlled experiments were carried out for understanding the reaction mechanism (Scheme 3). If 1 equiv. of K2HPO4·3H2O participated in the reaction with CH2Cl2, 4 was the major product in 28% yield, as well as 2a in 15% yield (eqn (1)). Increasing the amount of K2HPO4·3H2O to 2 equiv., the yield of 2a increased to 29%, while 4 was afforded in only 15%. When 4 reacted with CH2Cl2 under standard conditions for 6 h, 2a was obtained in 46% yield (eqn (2)). Prolonging the reaction time to 12 h, 4 furnished 2a in 71% yield. Surprisingly, 2a could not be detected without CH2Cl2, indicating that CH2Cl2 might be involved in the procedure of intramolecular attack. In addition, 4 was not observed when employing K3PO4·3H2O as the base in the course of the reaction.
On the basis of the results obtained, a plausible reaction mechanism was proposed and illustrated in Scheme 4. Initially, dehydration of salicylic acid (1a) formed a salt.32 Subsequently, A reacted with CH2Cl2, providing product 2a directly (Scheme 4).
In conclusion, we have developed a practical and efficient method to construct 4H-benzo[d][1,3]dioxin-4-one and its derivatives, which are important structural units in insecticides, and intermediates to synthesize multiple-substituted benzene. The catalyst- and additive-free conditions, commercial and cheap starting materials and short reaction time, make this transformation pretty green, practical and attractive. Further studies on the reaction mechanism and the synthetic applications are ongoing in our laboratory.
Acknowledgements
Financial support was provided by Program for Minjiang Scholar program (10BS216), Science Research Item of Science and Technology of Xiamen City (3502z201014) and Fundamental Research Fund of Huaqiao University.Notes and references
- P. A. Evans, M.-H. Huang, M. J. Lawler and S. Maroto, Nat. Chem., 2012, 4, 680 CrossRef CAS PubMed.
- M. A. Brodney, D. E. Johnson, A. Sawant-Basak, K. J. Coffman, E. M. Drummond, E. L. Hudson, K. E. Fisher, H. Noguchi, N. Waizumi and L. L. McDowell, J. Med. Chem., 2012, 55, 9240 CrossRef CAS PubMed.
- C. Burns, J. A. Del Vecchio, M. T. Bailey, R. B. Kulkarni, A. T. Faitg, H. S. Sherk, R. C. Black-Ledge, W. D. Rys, J. T. Lessen, A. J. Swestock, Y. Deng, T. Nitz, J. J. Reinhardt, A. H. Feng and K. Saha Ashis, WO 2004/041201 A2, 2004.
- D. Swinnen, C. Jorand-Lebrun, P. Gerber, J. Gonzalez and A. Bombrun, WO 2005/097773 A1, 2005.
- P. Desreumaux, S. Bellinvia, P. Chavate and S. Baroni, WO 2009/080821 A2, 2009.
- W.-B. Ho, L. Wright, S. Deng, E. Turtle and L. A. Flippin, WO 2009/100250 A1, 2009.
- Y. Takigawa, Y. Onda and K. Shimizu, WO 2012/091115 A1, 2012.
- G. A. Holloway, H. M. Hugel and M. A. Rizzacasa, J. Org. Chem., 2003, 68, 2200 CrossRef CAS PubMed.
- M. Oestreich, F. Sempere-Culler and A. B. Machotta, Angew. Chem., Int. Ed., 2005, 44, 149 CrossRef CAS PubMed.
- O. Soltani and J. K. De Brabander, Angew. Chem., 2005, 117, 1724 CrossRef.
- G. C. Clososki, C. J. Rohbogner and P. Knochel, Angew. Chem., Int. Ed., 2007, 46, 7681 CrossRef CAS PubMed.
- A. B. Machotta, B. F. Straub and M. Oestreich, J. Am. Chem. Soc., 2007, 129, 13455 CrossRef CAS PubMed.
- J. Garcia-Fortanet, J. R. Debergh and J. K. De Brabander, Org. Lett., 2005, 7, 685 CrossRef CAS PubMed.
- A. Ooguri, K. Nakai, T. Kurahashi and S. Matsubara, J. Am. Chem. Soc., 2009, 131, 13194 CrossRef CAS PubMed.
- J. K. Augustine, Y. A. Naik, A. B. Mandal, N. Chowdappa and V. B. Praveen, J. Org. Chem., 2007, 72, 9854 CrossRef CAS PubMed.
- K. D. Combrink, H. B. Gulgeze, K.-L. Yu, B. C. Pearce, A. K. Trehan, J. Wei, M. Deshpande, M. Krystal, A. Torri and G. Luo, Bioorg. Med. Chem. Lett., 2000, 10, 1649 CrossRef CAS.
- M. S. Deshpande, J. Wei, G. Luo, C. Cianci, S. Danetz, A. Torri, L. Tiley, M. Krystal, K.-L. Yu and S. Huang, Bioorg. Med. Chem. Lett., 2001, 11, 2393 CrossRef CAS.
- J. S. Rountree and P. V. Murphy, Org. Lett., 2009, 11, 871 CrossRef CAS PubMed.
- S. Kamisuki, S. Takahashi, Y. Mizushina, S. Hanashima, K. Kuramochi, S. Kobayashi, K. Sakaguchi, T. Nakata and F. Sugawara, Tetrahedron, 2004, 60, 5695 CrossRef CAS PubMed.
- N. Bajwa and M. P. Jennings, J. Org. Chem., 2006, 71, 3646 CrossRef CAS PubMed.
- T. Yoshino, I. Sato and M. Hirama, Org. Lett., 2012, 14, 4290 CrossRef CAS PubMed.
- R. G. Dushin and S. J. Danishefsky, J. Am. Chem. Soc., 1992, 114, 655 CrossRef CAS.
- S. W. Kang, C. M. Gothard, S. Maitra, Atia-tul-Wahab and J. S. Nowick, J. Am. Chem. Soc., 2007, 129, 1486 CrossRef CAS PubMed.
- U. Ichiro, Y. Yori and N. Satoshi, JP 2010/009435 A, 2010.
- D. T. Mowry, US 2510036 A, Dayton and Ohio, 1946.
- D. T. Mowry, W. H. Yanko and E. L. Ringwald, J. Am. Chem. Soc., 1947, 69, 2358 CrossRef CAS.
- P. Perlmutter and E. Puniani, Tetrahedron Lett., 1996, 37, 3755 CrossRef CAS.
- A. Hadfield, H. Schweitzer, M. P. Trova and K. Green, Synth. Commun., 1994, 24, 1025 CrossRef CAS.
- I. Shibuya, Y. Gama, M. Shimizu and M. Goto, Heterocycles, 2002, 57, 143 CrossRef CAS.
- K. Kulbitski, G. Nisnevich and M. Gandelman, Adv. Synth. Catal., 2011, 353, 1438 CrossRef CAS.
- K. Holmberg and B. Hansen, Tetrahedron Lett., 1975, 16, 2303 CrossRef.
- F. Lin, Q. Feng, X. Cui and Q. Song, RSC Adv., 2013, 3, 20246 RSC.
- M. Schlosser, Angew. Chem., Int. Ed., 2006, 45, 5432 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: See DOI: 10.1039/c4ra01651c |
| This journal is © The Royal Society of Chemistry 2014 |






