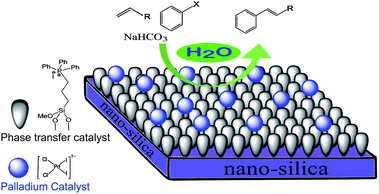
Simultaneous immobilization of a matrix containing palladium and phase transfer catalyst on silica nanoparticles: application as a recoverable catalyst for the Heck reaction in neat water†
Abdol R. Hajipour*ab and
Ghobad Azizib
aPharmaceutical Research Laboratory, Department of Chemistry, Isfahan University of Technology, Isfahan 84156, Islamic Republic of Iran. E-mail: haji@cc.iut.ac.ir; arhajipour@wisc.edu; Fax: +98 311 391 2350; Tel: +98 311 391 3262
bDepartment of Neuroscience, University of Wisconsin, Medical School, 1300 University Avenue, Madison, WI 53706-1532, USA
First published on 18th April 2014
Abstract
Simultaneous covalent anchoring of a phosphonium–palladium complex/phase transfer catalyst matrix on the surface of silica nanoparticles and the application of the resulting catalyst in the Heck reaction of a variety of different haloarenes in neat aqueous media is described.
Introduction
The palladium catalysed carbon–carbon bond forming reaction between aryl halides and olefins in the presence of a base (Heck reaction) is a powerful method for the synthesis of various organic molecules.1The usual palladium species used in the Heck reactions are palladium acetate, chloride or preformed triarylphosphine–palladium complexes.2 Despite the higher catalytic activities of homogeneous catalysts than their heterogeneous counterparts, as with all homogeneous catalysts, there are several problems such as separation, regeneration and reuse of the expensive catalyst from the product.3 These problems are of environmental and economic concern especially in large scale synthesis where large volumes of hazardous waste and high production costs are of great importance.4 These problems can be largely overcome by carrying out the reactions using heterogeneous-based catalysts and use of green solvents as reaction solvents. Heterogeneous catalysts will be promising alternatives, owing to their air-stability, recoverability and reusability.5
Despite all the developments on catalyst design, polar aprotic solvents such still remain the preferred medium for carrying out Heck reactions. Driven by environmental concerns, recently, much effort has been directed towards using water as solvent for this reaction. However, among the large number of palladium catalysed Heck reactions, there are a few examples have been reported that deal with Heck coupling in neat water without usage of any organic co-solvents.6
The use of water as solvent in organic synthesis is an important goal for the development of environmentally safe chemical processes. These unique characteristics include low-cost, non-flammable, non-toxic, high cohesive energy density, high dielectric constant and environmental compatibility.7,8
Furthermore, due to enhanced hydrophobic interactions and the enrichment of organic substrates in the local environment in aqueous media, water often exhibits profound effects on the rate and selectivity of organic reactions.9,10
However, the presence of water in reaction media reduces interaction of organic and water soluble substrates. Phase-transfer catalysis is a green approach for driving reactions involving these immiscible reactants.
Nevertheless, a practical problem associated with homogenous phase-transfer catalysts (PTC) is the difficulty of separation. The attachment of phase-transfer catalysts to insoluble supports such as magnetic nanoparticles, polymers and silica has afforded a solution to overcome this problem and greatly simplifies their use in many organic reactions.11–23 A recently developed strategy is reversibly transferring the catalyst between two immiscible phases in response to environment changes such as pH.24 Having previously developed palladium-supported ionic liquid catalysts via immobilization of palladium acetate on amorphous diethylaminopropylated silica with the aid of [bmim]PF6 by Hagiwara that catalyse various organic transformations,8,25–28 N-heterocyclic carbene palladium complex supported on ionic liquid-modified SBA-16 by Yang29 as a recyclable heterogeneous catalyst for the Suzuki and Heck reactions, N-heterocyclic carbene palladium/ionic liquid matrix immobilized on silica by Karimi30 for Heck reaction in NMP, and PdCl2–ionic liquid brush assembly on silica gel by Shi31,32 for Suzuki and Heck reaction in neat water.
We then envisioned a simultaneous grafting strategy for the preparation a matrix contain both palladium and phosphonium moieties on silica nanoparticles that could be applied to the Heck reaction of aryl halides.
With catalytic utility in mind, we designed a matrix of phosphonium iodides with flexible alkyl-chain tethers and dianion [PdCl2I2]2− species electrostatically interacted with two phosphonium cations. Tethered phosphonium iodides can act as PTC and palladium species serve as catalytically active centres for Heck reaction.
Results and discussion
We recently reported the application of palladium complexes with quaternary phosphonium cations in the Heck coupling reaction.33 Here, we are going to immobilize these types of palladium complexes covalently onto a solid support. Since the synergistic effects between the supported material and the reagent can lead to unexpectedly high activity, we wish to describe a novel catalyst, bearing the matrix containing both palladium and phase transfer (PT) catalyst supported on chemically-modified silica nanoparticles (Fig. 1). The use of this Pd/PT catalyst was investigated in the Heck reaction in neat water which, while being environmentally benign, rival conventional Heck reaction procedures in synthetic efficiency and operationalsim policy.This work describes the simultaneous covalent immobilization of the zwitterionic palladium complex/phase transfer catalyst matrix onto silica and its application in the Heck reaction in pure water as the reaction solvent.
The catalyst was prepared by the synthesis of quaternary phosphonium iodide structure followed by complexation with palladium chloride to form [PdCl2I2]2− derivative and simultaneous grafting of [PdCl2I2]2− on the surface of silica nanoparticles along with quaternary phosphonium iodides. The preparation procedure is shown in Scheme 1. The silane coupling agent triphenyl(3-trimethoxysilylpropyl)phosphanium iodide 1 was first synthesized by the reaction of triphenyl phosphine with the corresponding 3-(iodopropyl)trimethoxysilane in refluxing toluene.34 The phosphonium salt was obtained as a glassy pale yellow solid. Then, one equivalent of PdCl2 was allowed to react with sixteen equivalents of 1 under an inert atmosphere at reflux temperature for 2 h in dry CHCl3 to afford a dark purple solution. The resulting product was further reacted with SiO2 nanoparticles under reflux for 5 h to form the corresponding silica supported Pd/PTC matrix system (Fig. 1S in ESI†). The palladium loading was 0.024 mmol g−1 determined by atomic absorption analysis. Due to mass transfer limitations of the supported phase transfer catalyst, we choose silica nanoparticles for better dispersion than micron sized silica gel. Fig. 2S† shows the typical TEM image of the catalyst. The average diameter of the catalyst nanoparticles was about 12 nm and the size distribution was narrow. The infrared spectrum of the quaternary phosphonium modified silane coupling agent 1 and quaternary phosphonium modified nanosilica (Fig. 2 and 3 respectively) confirms the presence of triphenyl phosphine moiety on the silica bed. The infrared spectrum shows bands at 3065 (C–H phenyl ring), 1587 and 1486 (C–C phenyl), 746 and 690 cm−1 (C–H OOP) correspond to phenyl groups of triphenyl phosphine. No change in IR spectrum observed upon complexation with palladium (Fig. 2). 1H-NMR spectra of salt 1 (Fig. 3S†) shows five types of hydrogen and confirmed the structure of 1. Diffuse reflectance UV-Vis spectroscopy was employed for quaternary phosphonium/palladium modified nanosilica and compared with UV-Vis spectra of unsupported palladium complex 2 in CH2Cl2 solution. Both spectra exhibit absorption bands in the 240–280 nm regions (Fig. 5S and 6S). SEM-EDX analysis shows the presence of palladium, iodide and chloride in the catalyst matrix (Fig. 7S†). These results clearly indicate the successful covalent attachment of the above mentioned matrix onto the silica surface.
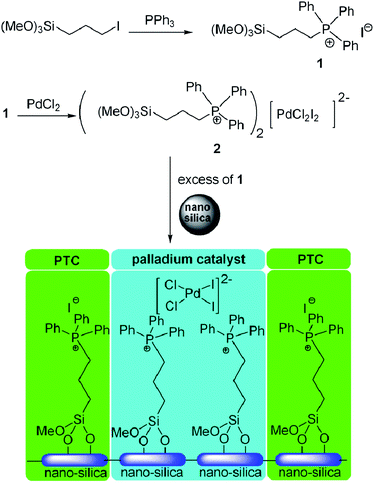 | ||
| Scheme 1 Synthesis process and immobilization of Pd/phase-transfer catalyst as a matrix on the silica nanoparticle. | ||
Reactivity of this immobilized Pd/PT catalyst was tested in the coupling reaction of iodobenzene and methylacrylate (MA) in pure water. In a typical reaction, equimolar amounts of iodobenzene and MA, 1.5 equivalents of NaHCO3, 5 mg of catalyst (0.012 mol% Pd) and 2 mL of water were charged to a 5 mL round bottom flask equipped with condenser. Under the present condition, NaHCO3 is completely soluble in water but the two other substrates are not. The mixture was heated to 100 °C for 12 hours. Under this condition, only 30% conversion obtained. By using the stainless steel reactor and 130 °C, the reaction proceeds completely in 5 hours. In order to find the optimal reaction conditions, several bases were tested for this reaction and NaHCO3 was determined to be the most effective. No conversion of iodobenzene to corresponding product was observed without catalyst and some degree of polymerization of MA occurred. The synthetic efficacy of this aqueous Heck reaction was then studied with a number of different aryl halides and olefins and the results shown in Table 1. Heck reaction of aryl iodides with MA and acrylic acid gave good yields of the corresponding products within 2–7 h at 130 °C. As expected, aryl iodides with an electron donating substituent considerably lowered the reaction rate.
| Entry | R′ | Olefin | X | Time (h) | Yield |
|---|---|---|---|---|---|
| a Reaction conditions: aryl halide (1 mmol), olefin (1 mmol), NaHCO3 (1.5 mmol), catalyst (5 mg, 0.00012 mmol Pd) and H2O at 130 °C.b Acrylic acid. | |||||
| 1 | H | MA | I | ||
| TON | TOF/h | ||||
| Run 1 | 7667 | 1533 | 5 | 92 | |
| Run 2 | 7917 | 1583 | 5 | 95 | |
| Run 3 | 7500 | 1500 | 5 | 90 | |
| Run 4 | 7249 | 1450 | 5 | 87 | |
| Run 5 | 7083 | 1417 | 5 | 85 | |
| Run 6 | 5333 | 1066 | 5 | 64 | |
| Total TON | 42![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 749 749 |
||||
| 2 | 4-NO2 | MA | I | 2 | 95 |
| 3 | 3-NO2 | MA | I | 2 | 95 |
| 4 | 4-OCH3 | MA | I | 7 | 88 |
| 5 | 4-CN | MA | I | 2 | 93 |
| 6 | 4-Cl | MA | I | 2 | 96 |
| 7 | 4-NO2 | MA | Br | 8 | 94 |
| 8 | 4-OCH3 | MA | Br | 24 | 72 |
| 9 | 4-CN | MA | Br | 3 | 96 |
| 10 | 4-NO2 | AAb | I | 2 | 95 |
| 11 | 4-OCH3 | AA | I | 8 | 80 |
| 12 | 4-CN | AA | I | 3 | 97 |
| 13 | 4-NO2 | AA | Br | 8 | 89 |
| 14 | 4-OCH3 | AA | Br | 24 | 77 |
| 15 | 4-CN | AA | Br | 5 | 92 |
| 16 | 4-Br | MA | Br | 24 | 63 |
Less reactive bromoarenes required even longer reaction times, the corresponding Heck product being obtained in 72–96% yield after 3–24 h. The reactivity of aryl bromides with electron-withdrawing substituent was higher than those with electrondonating substituent and was comparative with aryl iodides. Dibromobenzene also successfully diolefinated in 63% yields.
As drawn in Scheme 2, the PTC functions to draw inorganic base into solid phase (surface of catalyst) as a P+HCO3− ion pair, liberating Na+I− into aqueous phase. P+HCO3− then reacts with H–Pd–I intermediate to form P+I− and H2CO3.
 | ||
| Scheme 2 Phase transfer catalysis of Heck reaction catalysed by supported Pd/PTC matrix immobilized on silica nanoparticles. | ||
For practical applications of palladium heterogeneous catalysts, the reusability of catalyst is a very important factor. To clarify this issue, catalytic recycling experiments were carried out using Heck reaction of iodobenzene and MA as model reaction. After the completion of reaction, the catalyst could be conveniently and efficiently recovered from the reaction mixture by centrifugation, it can be used in the next run after washing with ethanol and acetone and charging with fresh substrates and then repeating the experiment. The recycled catalyst can be used at least five times before there is a significant drop in activity (Table 1 shows representative recycle runs). This high catalytic activity is clearly reflected by a high total turnover number up to 42![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 749 for 6 reuses.
749 for 6 reuses.
The catalytic activity of this catalyst compared with Pd/SiO2 (same palladium loading and reaction conditions) showed that in the presence of Pd/SiO2, after about 17 hours, 91% conversion obtained. Comparison with silica supported PdCl2–ionic liquid brush in neat water by Shi32 reveals that our catalyst exhibits better catalytic performance in terms of total TON (42![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 749 for six runs vs. 1483 for eight runs), reaction times (2 hours vs. 24 hours for reaction of 4-iodonitrbenzene and acrylic acid) and olefin equivalent (1 equiv. vs. 1.2 equiv.).
749 for six runs vs. 1483 for eight runs), reaction times (2 hours vs. 24 hours for reaction of 4-iodonitrbenzene and acrylic acid) and olefin equivalent (1 equiv. vs. 1.2 equiv.).
Comparison with homogeneous complex of palladium chloride and methyl triphenylphosphonium iodide reveals that the rate of the reaction in the presence of homogeneous complex is faster than immobilized one in the same reaction conditions (2 hours vs. 5 hours for iodobenzene and MA). As we described in our previous work,33 decomposition of palladium–phosphonium complex during Heck reaction forms palladium nanoparticles and quaternary phosphonium cations that act as stabilizer for palladium nanoparticles. In the presence of that homogeneous catalyst, iodobenzene reacts in DMF as solvent in only 12 minutes.
Since after first use, palladium nanoparticles form on the surface of the silica particles, a comparative experiment with palladium nanoparticles supported on nano-SiO2 also performed (same reaction conditions in Table 1). In the presence of methyl triphenylphosphonium iodide as homogeneous PTC, reaction was completed after 7 hours, but in the absence of PTC, after 5 hours, only 47% conversion obtained.
After the first run, the purple colour of the catalyst turned to black. This change indicates the formation of metallic palladium on the surface of silica nanoparticles (Fig. 4). The formation of metallic palladium was confirmed by powder XRD analysis. In the XRD spectrum of the original catalyst (Fig. 5, up), it could be seen that only a broad maximum at 2θ of 22.18 is assigned to the amorphous silica bed. This confirms the presence of highly dispersed palladium(II) deposited on the silica matrix. Moreover, apart from the original peak, the appearance of the new peaks at 2θ of 39.88°, 46.12°, and 67.95°, which corresponding to (111), (200), and (220) crystalline planes of palladium was observed in the spectrum (Fig. 5), indicating that metallic palladium phase exists in the form of palladium(0). The extent of palladium leaching for the reaction of MA and iodobenzene was measured by removing the solid catalyst from hot reaction solution by centrifugation after the reaction completion, and analysing the resulting solutions by ICP-MS. Catalyst was examined after the first reuse. Palladium content was about 0.1 ppm. This indicates that 1.2% (0.0003 mg of total 0.0254 mg supported Pd) was dissolved in reaction solution.
Conclusions
In conclusion, the simultaneous immobilization of phosphonium salt and palladium complex on silica nanoparticles provided an efficient Pd/PTC matrix applied for Heck reaction in pure water that could easily be recovered and recycled.Acknowledgements
We gratefully acknowledge the funding support received for this project from the Isfahan University of Technology (IUT), IR Iran (A.R.H.), and further financial support from Center of Excellency in Sensor and Green Chemistry Research (IUT) is gratefully acknowledged.Notes and references
- R. F. Heck, Acc. Chem. Res., 1979, 12, 146–151 CrossRef CAS.
- J. H. Clark, D. J. Macquarrie and E. B. Mubofu, Green Chem., 2000, 2, 53–56 RSC.
- C. E. Garrett and K. Prasad, Adv. Synth. Catal., 2004, 346, 889–900 CrossRef CAS.
- A. J. Butterworth, J. H. Clark, P. H. Walton and S. J. Barlow, Chem. Commun., 1996, 1859–1860 RSC.
- Q. Du, W. Zhang, H. Ma, J. Zheng, B. Zhou and Y. Li, Tetrahedron, 2012, 68, 3577–3584 CrossRef CAS PubMed.
- M. Lamblin, L. Nassar-Hardy, J.-C. Hierso, E. Fouquet and F.-X. Felpin, Adv. Synth. Catal., 2010, 352, 33–79 CrossRef CAS.
- L. Botella and C. Nájera, Tetrahedron Lett., 2004, 45, 1833–1836 CrossRef CAS PubMed.
- H. Hagiwara, Y. Sugawara, T. Hoshi and T. Suzuki, Chem. Commun., 2005, 2942–2944 RSC.
- T. Kurahashi, H. Shinokubo and A. Osuka, Angew. Chem., Int. Ed., 2006, 45, 6336–6338 CrossRef CAS PubMed.
- F. Zhao, M. Shirai and M. Arai, J. Mol. Catal. A: Chem., 2000, 154, 39–44 CrossRef CAS.
- A. Akelah, Eur. Polym. J., 1982, 18, 559–561 CrossRef CAS.
- R. Annunziata, M. Benaglia, M. Cinquini, F. Cozzi and G. Tocco, Org. Lett., 2000, 2, 1737–1739 CrossRef CAS PubMed.
- S. Desikan and L. K. Doraiswamy, Chem. Eng. Sci., 2000, 55, 6119–6127 CrossRef CAS.
- L. Li, J. Shi, J. Yan, H. Chen and X. Zhao, J. Mol. Catal. A: Chem., 2004, 209, 227–230 CrossRef CAS PubMed.
- Q. Shi, Y.-J. Lee, M.-J. Kim, M.-K. Park, K. Lee, H. Song, M. Cheng, B.-S. Jeong, H.-g. Park and S.-s. Jew, Tetrahedron Lett., 2008, 49, 1380–1383 CrossRef CAS PubMed.
- K. Tomita and T. Ono, J. Polym. Sci., Part A: Polym. Chem., 2009, 47, 762–770 CrossRef CAS.
- M. Tomoi, E. Nakamura, Y. Hosokawa and H. Kakiuchi, J. Polym. Sci., Polym. Chem. Ed., 1985, 23, 49–61 CrossRef CAS.
- P. Tundo and P. Venturello, Tetrahedron Lett., 1980, 21, 2581–2584 CrossRef CAS.
- P. Tundo and P. Venturello, J. Am. Chem. Soc., 1981, 103, 856–861 CrossRef CAS.
- P. A. Vivekanand and M.-L. Wang, Catal. Commun., 2012, 22, 6–12 CrossRef CAS PubMed.
- M. Wang and H. Zong, Polym. Adv. Technol., 1996, 7, 35–38 CrossRef CAS.
- Z. Wang, L. Xu, Z. Mu, C. Xia and H. Wang, J. Mol. Catal. A: Chem., 2004, 218, 157–160 CrossRef CAS PubMed.
- H.-M. Yang and C.-C. Huang, Catal. Lett., 2009, 128, 235–242 CrossRef CAS.
- H. Wang, H. Yang, H. Liu, Y. Yu and H. Xin, Langmuir, 2013, 29, 6687–6696 CrossRef CAS PubMed.
- H. Hagiwara, K. H. Ko, T. Hoshi and T. Suzuki, Chem. Commun., 2007, 2838–2840 RSC.
- H. Hagiwara, T. Kuroda, T. Hoshi and T. Suzuki, Adv. Synth. Catal., 2010, 352, 909–916 CrossRef CAS.
- H. Hagiwara, T. Nakamura, T. Hoshi and T. Suzuki, Green Chem., 2011, 13, 1133–1137 RSC.
- H. Hagiwara, Y. Sugawara, K. Isobe, T. Hoshi and T. Suzuki, Org. Lett., 2004, 6, 2325–2328 CrossRef CAS PubMed.
- H. Yang, X. Han, G. Li and Y. Wang, Green Chem., 2009, 11, 1184–1193 RSC.
- B. Karimi and D. Enders, Org. Lett., 2006, 8, 1237–1240 CrossRef CAS PubMed.
- X. Shi, X. Han, W. Ma, J. Fan and J. Wei, Appl. Organomet. Chem., 2012, 26, 16–20 CrossRef CAS.
- J.-F. Wei, J. Jiao, J.-J. Feng, J. Lv, X.-R. Zhang, X.-Y. Shi and Z.-G. Chen, J. Org. Chem., 2009, 74, 6283–6286 CrossRef CAS PubMed.
- A. R. Hajipour and G. Azizi, Synlett, 2013, 24, 254–258 CrossRef CAS PubMed.
- M. Kawamura and K. Sato, Chem. Commun., 2006, 4718–4719 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Copy of NMR, EDX, and UV spectra and TEM image. See DOI: 10.1039/c4ra01634c |
| This journal is © The Royal Society of Chemistry 2014 |