Bioconversion of coal: new insights from a core flooding study†
Abstract
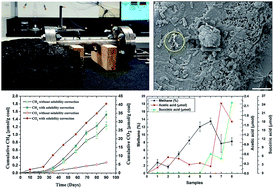
A pressurized core flooding experiment was performed to better understand in situ coal bioconversion processes. The core flooding experiment was conducted using a biaxial core holder packed with subbituminous coal particles (250–150 μm grain size) obtained from the Highvale mine in Alberta, Canada. The coal pack was inoculated with a methanogenic microbial culture enriched from coal and was continuously flooded with mineral salt medium and an organic carbon/nitrogen nutrient supplement (tryptone). The changes in the physical properties of the coal pack during the core flooding suggested coal bioconversion to methane under the experimental conditions. Colonization and bioconversion of coal by microbes was evident from the change in core permeability and presence of metabolites and gas (CH4 and CO2) in the effluent. A total of 1.52 μmol of CH4 was produced per gram of coal during the 90 days experiment at 22 °C. Signature metabolites consistent with anaerobic biodegradation of hydrocarbons, e.g., carboxylic acids, were identified in effluent samples throughout incubation. The transient nature of metabolites in effluent samples supports fermentation of coal constituents and nutrient supplement to simple molecules such as acetic acid, which served as a substrate for methanogenesis during the bioconversion process. Accumulation of carboxylic acids such as succinic acid in the effluent also demonstrates that the coal bioconversion process may be used for extraction of other value-added products apart from CH4 generation. Importantly, results presented here suggest that coal bioconversion by biostimulation under reservoir conditions is a scalable technology with potential for energy generation and for overall reduction of greenhouse gas emissions.

 Please wait while we load your content...
Please wait while we load your content...