Facile method to synthesize silver nanoparticles on the surface of hollow glass microspheres and their microwave shielding properties
Abstract
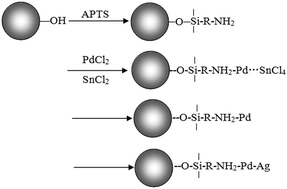
In this paper, a facile method for the fabrication of hollow glass microspheres–Ag composite particles with core–shell structures is investigated. Ag-coated hollow glass microspheres with raspberry morphology have been prepared by an in situ composite technique in the presence of polyvinylpyrrolidone (PVP). The as-prepared composite particles are characterized by XRD, SEM, EDS, TEM, IR, and XPS. The results show that 3-aminopropyltriethoxysilane (APTS) coupling can remarkably improve the adhesion between the palladium colloid particles and the surface of hollow glass microspheres, increasing the amount of active sites on such surfaces. With the increase in pH value, the equilibrium of the [Ag(NH3)2]+ solution is destroyed, which is beneficial to the output of Ag nanoparticles. The shell thickness of silver-coated hollow glass microspheres increases from 30 nm to 52 nm with an increase in the concentration of the [Ag(NH3)2]+ solution. The shielding property of the composite particles increases gradually and shows an obvious increase in the range of 30% to 35% with an increase in the volume fraction of the composite particles. When the volume fraction of the filler reaches 35%, electromagnetic shielding effectiveness is between 70 dB and 80 dB with the frequency of electromagnetic wave ranging from 2 GHz to 12 GHz. These results indicate that the Ag-coated glass microsphere's core–shell particles could have extensive applications in the electromagnetic compatibility field.

 Please wait while we load your content...
Please wait while we load your content...