Coumarin–hemicyanine conjugates as novel reaction-based sensors for cyanide detection: convenient synthesis and ICT mechanism†
Abstract
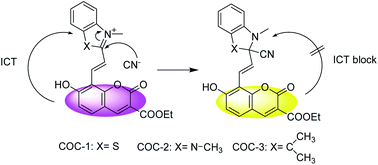
Using intramolecular charge transfer (ICT) as a signaling mechanism, a series of hybrid coumarin–hemicyanine compounds were synthesized as chemosensors for cyanide detection by taking advantage of cyanide's strong affinity toward the polarized C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond of the hemicyanine group. Structure identification of the compounds was confirmed by 1H NMR, 13C NMR, 1H-1H COSY, HSQC, IR, and HRMS spectroscopy. Multiple sensory signals are available and can be used for both qualitative monitoring and quantitative determination of cyanide, including high-contrast visual color change, fluorescence quenching and enhancement.
N bond of the hemicyanine group. Structure identification of the compounds was confirmed by 1H NMR, 13C NMR, 1H-1H COSY, HSQC, IR, and HRMS spectroscopy. Multiple sensory signals are available and can be used for both qualitative monitoring and quantitative determination of cyanide, including high-contrast visual color change, fluorescence quenching and enhancement.

 Please wait while we load your content...
Please wait while we load your content...