Synthesis of WO3·H2O nanoparticles by pulsed plasma in liquid
Liliang Chen†a,
Tsutomu Mashimo*a,
Hiroki Okuderab,
Chihiro Iwamotoc and
Emil Omurzakd
aInstitute of Pulsed Power Science, Kumamoto University, Kumamoto 860-8555, Japan. E-mail: mashimo@gpo.kumamoto-u.ac.jp; Fax: +81 96 342 3293; Tel: +81 96 342 3295
bFaculty of Natural System, Institute of Science and Engineering, Kanazawa University, Kanazawa 920-1192, Japan
cFaculty of Engineering, Kumamoto University, Kumamoto 860-8555, Japan
dPriority Organization for Innovation and Excellence, Kumamoto University, Kumamoto 860-8555, Japan
First published on 6th June 2014
Abstract
Pure orthorhombic-phase WO3·H2O nanoparticles with sizes of about 5 nm were synthesized by pulsed plasma in deionized water, in which tungsten electrodes provide the source of tungsten and the water is the source of oxygen and hydrogen. The quenching effect and liquid environment inherent in this pulsed plasma in liquid method resulted in ultra-small particles with lattice lengths (a = 5.2516 Å, b = 10.4345 Å, c = 5.1380 Å) larger than those of reference lattices. The emission lines of W I atoms, W II ions and H I atoms were observed by an optical emission spectrum in order to gather information on the synthetic mechanism. These nanoparticles showed higher absorption in the visible region than did ST-01 TiO2 and Wako WO3 nanoparticles. The WO3·H2O nanoparticles displayed more activity in the photocatalytic test than did the commercial TiO2 sample (ST-01). Also, the absorption edge of WO3·H2O shifted to longer wavelengths in the UV-Vis absorption pattern relative to that of the anhydrous tungsten oxide.
1 Introduction
There is a keen need for new forms of ‘green’ energy. The development of solar energy, such as that produced using photocatalysts, will have great long-term benefits since this form of energy is affordable, inexhaustible and clean. The most prevalently used photocatalyst, TiO2, is relatively inefficient, however, at utilizing visible light.1 Numerous efforts to modify its band structure by doping have not been very successful because dopants usually act as recombination centers between the photogenerated electrons and holes, which markedly reduce the photocatalytic activity.2As a potential substitute, WO3, which possesses a small band-gap energy of 2.4–2.8 eV, stable physicochemical properties and resilience to photocorrosion effects,3 has strong photocatalytic activity in the visible-light region. However, its low conduction band level limits the photocatalyst to react with electron acceptors4 and then increases the recombination of photogenerated electron–hole pairs. Thus, much effort has been focused on the particles modified with other components, such as Ag.5,6
Although less studied than anhydrous tungsten oxide, the association of water with tungsten oxide to form the WO3·H2O hydrate is considered to be important for the chemical properties of the resulting molecular species, and has been shown to cause a change in optical absorption.7 This indicates that hydration may modify the band structures of WO3, and thus may alter its photocatalytic properties for specific applications, even though the hydrate has lower photocatalytic efficiency than that of unhydrated WO3.
In this study, we carried out and analyzed the results of a one-step synthesis of WO3·H2O nanoparticles, using the pulsed plasma in liquid method.8,9
2 Experimental
As shown in Fig. 1, two metallic electrodes with a diameter of 5 mm and 99.95% tungsten purity (Rare Metallic Co., Ltd.) (hereafter called simply “tungsten electrodes”) were submerged in degassed deionized water in a quartz beaker without adding conducting salts (pH value is about 7). Pulses with the same single-pulse duration of about 15 μs, as shown in Fig. 2, were generated between the two rod tips at a voltage of 100 V and current of 50 A when the electrolysis of water occurred. This process utilized deionized water as a source of oxygen and hydrogen, and tungsten electrodes as the source of tungsten. The whole synthetic system only contained the elements W, O and H (from tungsten rods and water) without contamination from other metal cations. The microsecond duration of pulsed plasma and the surrounding cool liquid helped to quench the growth of the small-sized nanoparticles. After a one-hour reaction, the yellow-green nanoparticles were carefully collected after drying in air at 120 °C for 2 hours. | ||
| Fig. 1 Schematic of the system to generate pulsed plasma in deionized water by use of two tungsten electrodes. | ||
 | ||
| Fig. 2 Plot of current versus time for a single-pulse duration generated by the pulsed plasma in liquid system. | ||
Nanoparticle phase purity and structure were determined by X-ray diffraction (XRD) (Rigaku RINT 2000/PC) using Cu Kα radiation (40 kV, 200 mA). Crystal parameters were calculated by Rietveld refinement. Morphology and microstructural characterization of prepared samples were observed using high-resolution transmission electron microscopy (HRTEM) (Philips Tecnai F20). The powders for HRTEM were prepared by putting them into ethanol and deaggregating them by sonication for 30 min. Optical emission spectra were obtained using an optical probe placed adjacent to the beaker, and data were transmitted via an optical fiber to an SEC2000-UV-VIS spectrometer. A HITACHI F-2500 luminescence spectrophotometer was used to evaluate photocatalytic activity by the photodecomposition of acetaldehyde into CO2. A Tedlar bag (AS ONE Co. Ltd.) was used as the photo-reactor vessel with a volume of 125 cm3. A mass of 100 mg of WO3·H2O powder was spread evenly on the bottom of a glass dish (area: 9.6 cm2 = irradiation area), which was placed in the reaction vessel described above. Five hundred ppm of acetaldehyde was prepared in the vessel by injection of saturated gaseous acetaldehyde. Irradiation was conducted at room temperature after equilibrium between the gaseous and adsorbed acetaldehyde had been reached, which was ascertained by monitoring the concentration by a gas chromatograph approximately every 30 minutes. Methane and CO byproducts were negligible. The excited light source was an LED lamp with the parameters of 455 nm and 1 mW m−2 at room temperature. The photocatalytic results were compared with a commercial TiO2 nanoparticle (ST-01, anatase, Ishihara Sangyo Kaisha, Ltd.) and commercial WO3 (Wako Pure Chemical Industries, Ltd.). UV-Vis spectra of the synthesized sample, ST-01 and Wako WO3 were taken using a JASCO V-550 UV/VIS spectrometer.
3 Results and discussion
3.1. Characterization of WO3·H2O nanoparticles
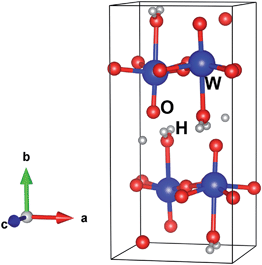
The crystal structure of the sample was checked by XRD (Fig. 3). The crystal yielded diffraction peaks at 2θ = 16.5, 19.3, 23.8, 25.7, 30.5, 33.4, 34.2, 35.0, 37.7, 38.9, 42.8, 45.9, 49.2, 52.8, 54.3, 56.3, 62.8 and 66.2°, corresponding well with the JCPDS card number 43-0679. This diffraction confirmed that the obtained nanoparticles were the WO3·H2O hydrate. The absence of metallic tungsten indicated the high purity of the WO3·H2O nanoparticles synthesized by the pulsed plasma in liquid method.Table 1 lists Rietveld refinement parameters of WO3·H2O obtained by pulsed plasma in deionized water, comparing them with Szymanski's data10 and reference data in PDF# 43-0679. The crystallographic structure of the synthesized WO3·H2O sample is well refined in the orthorhombic Pnmb space group (see Fig. 4 and 5) in the region of 14 to 90 degrees. Observed data are indicated by dots, and the calculated profile is indicated by a solid line. Short vertical bars below the pattern represent the positions of all possible Bragg reflections, and the line below the short vertical bars represents the difference between the observed and calculated patterns. Because the hydrogen atoms are very light, they were neglected and not calculated in this refinement, and were just added into the model of the structure for creating the image. Compared with the cell parameters from the other sources, the crystal parameters of the WO3·H2O nanoparticles prepared by pulsed plasma in deionized water are the largest. In our experiment, the plasma was produced in liquid, and water was the only source of oxygen. It was highly possible that the synthesized nanocrystals contain oxygen vacancies which tend to increase the lattice lengths.11 The quenching effect by the surrounding cool liquid during plasma synthesis may also help to inhibit the crystal growth and result in lattice expansion of the nanocrystals.12 Furthermore, the atomic positions are shifted a little in the plasma sample (Table 1), which indicates that the pulsed plasma in liquid method can introduce structural distortion in the synthesized nanoparticles.
| Sample | Plasma sample | Szymanski's data8 | PDF#43-0679 |
|---|---|---|---|
| Space group | Pnmb | Pnmb | Pnmb |
| Lattice parameter | |||
| a (Å) | 5.2516(8) | 5.249(2) | 5.238 |
| b (Å) | 10.7345(1) | 10.711(5) | 10.704 |
| c (Å) | 5.1380(7) | 5.133(2) | 5.12 |
| W | |||
| Position | 4c | 4c | 4c |
| x | 0.25 | 0.25 | |
| y | 0.2228(2) | 0.2209(8) | |
| z | 0.0123(2) | −0.0037(3) | |
| O1 | |||
| Position | 4c | 4c | 4c |
| x | 0.25 | 0.25 | |
| y | 0.4293(2) | 0.436(2) | |
| z | 0.0620(9) | 0.075(4) | |
| O2 | |||
| Position | 4c | 4c | 4c |
| x | 0.25 | 0.25 | |
| y | 0.0680(2) | 0.066(2) | |
| z | −0.0578(9) | −0.064(4) | |
| O3 | |||
| Position | 8d | 8d | 8d |
| x | 0.4626(8) | 0.495(8) | |
| y | 0.2555(3) | 0.227(2) | |
| z | 0.2864(9) | 0.249(5) | |
| T overall | 0.046758 | ||
| Profile R factors | |||
| GOF | 1.72 | ||
| Rp | 14.52 | ||
| Rwp | 18.32 | ||
 | ||
| Fig. 4 Rietveld refinement plot of WO3·H2O nanoparticles by pulsed plasma in deionized water using the orthorhombic space group Pnmb. | ||
High energy-density plasma commonly increases the synthesis temperature, leading to nanoparticle growth, but low energy-density plasma reduces the production rate. Thus, to optimize the balance between them, experimental parameters such as single-pulse duration and voltage were adjusted in order to prepare uniform small-sized WO3·H2O nanoparticles in large amounts. Besides, the synthetic environment of the pulsed plasma in liquid method helps to disperse nanoparticles in the liquid. As shown in Fig. 6, the morphology and particle size were investigated by HRTEM. The images indicated that very small orthorhombic WO3·H2O nanoparticles with sizes of about 5 nm were obtained by pulsed plasma in deionized water. The calculated size according to the Scherer equation from the peaks of the XRD patterns was about 250 um. Because we dried the particles before carrying out the XRD experiments, the results here are considered to be due to the aggregation of particles. The crystal parameters of WO3·H2O according to PDF card #43-0679 are 5.238 Å × 10.704 Å × 5.12 Å 〈90.0 × 90.0 × 90.0〉. As shown for the example in Fig. 6a, some particles were only about 1–2 nm in diameter, and contained only 1 or 2 unit cells. We conclude that some nanoparticles were nearly as small as the unit cell.13
 | ||
| Fig. 6 High-resolution TEM images of WO3·H2O nanoparticles by pulsed plasma in deionized water. (a) Image of a WO3·H2O crystal with an ultra-small size. | ||
Fig. 7 shows the energy-dispersive X-ray spectroscopy (EDX) pattern of a plasma sample, in which the element Cu is due to the HRTEM grid and the carbon peak source is the membrane covering the Cu grid. There were no other contaminating elements. The atomic percentages of W and O were 21.06% and 78.93%, respectively, closely fitting with the ratio of these two elements in WO3·H2O, which is equal to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.
4.
3.2. Mechanism of formation
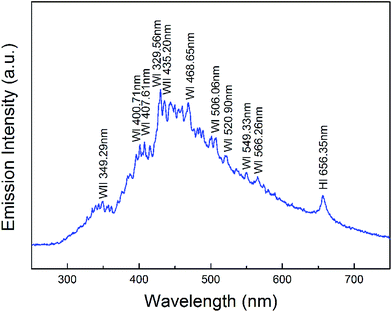
The optical emission spectrum of the pulsed plasma during the experiment is shown in Fig. 8. The spectral lines were identified using reference data. The coexistence of atoms W I, ions W II, and atoms H I, apparent from the optical emission spectrum, allows elucidation of the mechanism of formation of the WO3·H2O nanoparticles as follows: it is assumed that, first, very hot plasma with extreme energy ablates the tungsten metal in the tips of the rods to form very active tungsten atoms W I; simultaneously, the deionized water is decomposed by plasma into oxygen and hydrogen. Soon after, the atoms W I in the plasma discharge zone lose electrons and transform into ions W II, which then react with oxygen and hydrogen to quickly form a WO3·H2O molecular complex. When these complexes aggregate into crystallites and are quenched suddenly by the surrounding cool liquid, ultra-small WO3·H2O nanoparticles appear. According to the Balmer series, there should be additional emission lines due to H atoms, but these lines at about 410 nm, 430 nm, 490 nm, are masked by the strong emission peaks of W atoms.3.3. Photocatalytic properties and UV absorption
Photocatalytic properties of pure tungsten hydrate has not been garnering much attention. In the published literature, most of the reported hydrates were mixed with other components such as WO3 and Ag,6,14 and the hydrates that were investigated were usually in the forms WO3·0.33H2O and WO3·0.5H2O.15–17 Only recently, Q. Zeng et al.18 reported the photocatalytic properties of pure WO3·H2O hollow spheres. However, the synthesized spheres only showed high photocatalytic efficiency in acid solutions. Fig. 9 shows the dependence of the CO2 evolution rate on the decomposition of acetaldehyde under visible-light irradiation over pure WO3·H2O samples synthesized by pulsed plasma method. The irradiated wavelength was controlled by using various cut-off filters. The photocatalytic activity of WO3·H2O nanoparticles irradiated by visible light at 455 nm was higher than the photocatalytic activity of commercial TiO2 samples (ST-01) under any conditions. After 720 min, the amount of CO2 generated from the plasma sample is about 28.4% larger than that from ST-01.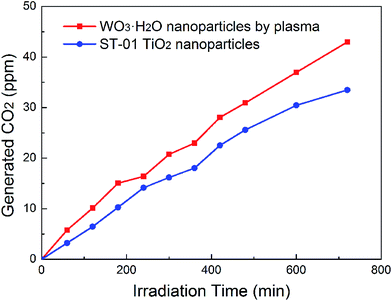 | ||
| Fig. 9 Photocatalytic properties of WO3·H2O nanocrystals grown in deionized water by pulsed plasma, compared with that of a commercial TiO2 nanoparticle (ST-01). | ||
In order to explain the photocatalytic behavior of WO3·H2O nanoparticles made using plasma and compare their light absorption ability to that of ST-01 and of commercial Wako WO3, UV-Vis absorption spectra (Fig. 10) were measured for these three species. The obvious red shift of the absorption edge and higher absorbance of the WO3·H2O sample in the visible-light region, compared with that of the ST-01 TiO2 sample, indicate the higher absorption and better photocatalytic properties of the WO3·H2O sample. Furthermore, the shift in the absorption edge from about 440 nm for anhydrous tungsten oxide to near 480 nm for the WO3·H2O sample shows that the band structure can be modified by hydration.
 | ||
| Fig. 10 UV-Vis absorption spectra of WO3·H2O nanoparticles synthesized by pulsed plasma in deionized water compared with that of a commercial TiO2 nanoparticle (ST-01) and of commercial WO3 (Wako). | ||
4 Conclusion
Pure WO3·H2O nanoparticles were prepared in a one-step synthesis by a pulsed plasma in deionized water method using two tungsten rods. The lattice of the sample was expanded relative to those of references, perhaps due to the quenching effect by the surrounding cool liquid during the preparation and the in-liquid environment for the synthesis. The mechanism of formation of the WO3·H2O particles was analyzed using an emission spectrum. These nanoparticles, with the ultra-small particle size of about 5 nm, exhibit higher absorption than do TiO2 (ST-01) and Wako WO3. The red shift of the absorption edge in the UV-Vis absorption pattern of the plasma-synthesized sample, compared with the absorption edge of the anhydrous tungsten oxide, indicates the possible band modification and the potential applications of WO3·H2O nanoparticles in the solar energy field.Acknowledgements
This work is supported by the China Scholarship Council and the Global Center of Excellence Program of Japan.References
- Z. Xiong, L. L. Zhang, J. Ma and X. S. Zhao, Chem. Commun., 2010, 46, 6099–6101 RSC.
- L. Z. Zhang, I. Djerdj, M. Cao, M. Antonietti and M. Niederberger, Adv. Mater., 2007, 19, 2083–2086 CrossRef CAS.
- G. R. Bamwenda and H. Arakawa, Appl. Catal., A, 2001, 210, 181–191 CrossRef CAS.
- R. Abe, K. Sayama and H. Sugihara, J. Phys. Chem. B, 2005, 109, 16052–16061 CrossRef CAS PubMed.
- Z. Wang and G. Chumanov, Adv. Mater., 2003, 15, 1285–1289 CrossRef CAS.
- P. Wang, B. Huang, X. Qin, X. Zhang, Y. Dai and M. Whangbo, Inorg. Chem., 2009, 48, 10697–10702 CrossRef CAS PubMed.
- K. O. Iwu, A. Galeckas, P. Rauwel, A. Y. Kuznetsov and T. Norby, J. Solid State Chem., 2012, 185, 245–252 CrossRef CAS PubMed.
- E. Omurzak, J. Jasnakunov, N. Mairykova, A. Abdykerimova, A. Maatkasymova, S. Sulaimankulova, M. Matsuda, M. Nishida, H. Ihara and T. Mashimo, J. Nanosci. Nanotechnol., 2007, 7, 3157–3159 CrossRef CAS PubMed.
- L. Chen, T. Mashimo, E. Omurzak, H. Okudera, C. Iwamoto and A. Yoshiasa, J. Phys. Chem. C, 2011, 115, 9370–9375 CAS.
- J. T. Szymanski and A. C. Roberts, Can. Mineral., 1984, 22, 681–688 CAS.
- F. Zhang, S. W. Chan, J. E. Spanier, E. Apak, Q. Jin, R. D. Robinson and I. P. Herman, Appl. Phys. Lett., 2002, 80, 127–129 CrossRef CAS PubMed.
- L. Chen, P. Fleming, V. Morris, J. D. Holmes and M. A. Morris, J. Phys. Chem. C, 2010, 114, 12909–12919 CAS.
- E. Salje, Acta Crystallogr., Sect. A: Cryst. Phys., Diffr., Theor. Gen. Crystallogr., 1977, 33, 574–577 CrossRef.
- M. S. Bazarjani, M. Hojamberdiev, K. Morita, G. Zhu, G. Cherkashinin, C. Fasel, T. Herrmann, H. Breitzke, A. Gurlo and R. Riedel, J. Am. Chem. Soc., 2013, 135, 4467–4475 CrossRef PubMed.
- L. Zou, Q. Liu, Q. Zhong, X. Bai and L. Dong, Adv. Mater. Res., 2009, 60–61, 480–485 CrossRef CAS.
- X. He, C. Hu, Q. Yi, X. Wang, H. Hua and X. Li, Catal. Lett., 2012, 142, 637–645 CrossRef CAS.
- X. Wang, X. Meng, M. Zhong, F. Wu and J. Li, Appl. Surf. Sci., 2013, 282, 826–831 CrossRef CAS PubMed.
- Q. Zeng, Y. Zhao, J. Zhao, X. Hao, Y. Lu, J. Guo, Y. Song, F. Gao and Z. Huang, Cryst. Res. Technol., 2013, 48, 334–343 CrossRef CAS.
Footnote |
| † Present address: The Peac Institute of Multiscale Sciences, Chengdu, Sichuan, 610207, China. |
| This journal is © The Royal Society of Chemistry 2014 |