Synthesis of graphene from natural and industrial carbonaceous wastes
Abstract
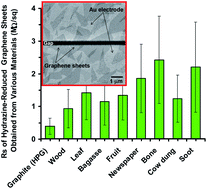
Graphene oxide (GO) and reduced graphene oxide (rGO) sheets have usually been synthesized through Hummers' method by using highly pure graphite (HPG) as the main starting material. However, HPG can be relatively expensive for mass production of high-quality graphene. In this work, a general method for synthesis of high-quality GO and rGO sheets from various natural and industrial carbonaceous wastes such as vegetation wastes (wood, leaf, bagasse, and fruit wastes), animal wastes (bone and cow dung), a semi-industrial waste (newspaper), and an industrial waste (soot powders produced in exhaust of diesel vehicles) was developed. Based on atomic force microscopy, Raman spectroscopy, X-ray photoelectron spectroscopy, and current–voltage characteristics of the synthesized sheets, the single- and multi-layer properties, chemical state, carbonaceous structure, and electrical properties of the graphene sheets synthesized from various waste materials (with ≤4-monolayer thicknesses and electrical sheet resistance of ∼105 MΩ sq−1 for GO and ∼1 MΩ sq−1 for rGO sheets) were found to be nearly independent of the starting materials used; moreover, they were comparable to those of the high-quality graphene sheets achieved using HPG. These results provide a possible route for inexpensive mass production of high-quality graphene sheets from natural and industrial carbonaceous wastes.

 Please wait while we load your content...
Please wait while we load your content...