Design and fabrication of a novel superhydrophobic surface based on a copolymer of styrene and bisphenol A diglycidyl ether monoacrylate†
Abstract
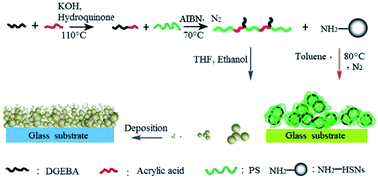
The copolymer of styrene and bisphenol A diglycidyl ether monoacrylate (PS-co-AADGEBA) was synthesized by three steps from raw materials, and then it was used to fabricate a superhydrophobic surface via a phase separation method. In particular the superhydrophobic surface not only exhibits superhydrophobicity towards water and corrosive liquids, including acidic and basic solutions, but also shows good transparency, improved robustness and excellent aging resistance. Furthermore, the PS-co-AADGEBA was also successfully grafted onto the surface of amino-functionalized hollow silica nanospheres (HSNs-NH2), then a (PS-co-AADGEBA)-g-(HSNs-NH2) nanocomposite superhydrophobic surface was prepared. The obtained surfaces are promising materials for numerous potential application fields, including electronics, biomedical, martial and defense-related areas.

 Please wait while we load your content...
Please wait while we load your content...