In-plane mesoporous graphene oxide nanosheet assembled membranes for molecular separation†
Abstract
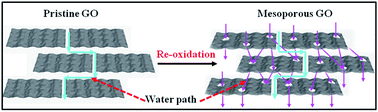
In-plane mesoporous graphene oxide (GO) sheets were prepared by a re-oxidation process and subsequently assembled into lamellar membranes. The in-plane pores significantly shortened the mass transport paths, resulting in 2–3 times higher water permeance than that of the pristine GO membrane, but continued to reject 3 nm molecules.

 Please wait while we load your content...
Please wait while we load your content...