DOI:
10.1039/C4RA01491J
(Paper)
RSC Adv., 2014,
4, 21918-21923
Dissolution behavior of deacetylated konjac glucomannan in aqueous potassium thiocyanate solution at low temperature
Received
20th February 2014
, Accepted 23rd April 2014
First published on 29th April 2014
Abstract
Deacetylation adversely affected the solubility of konjac glucomannan (KGM) in water; however, KGM with different degrees of deacetylation could be well dissolved in 9 wt% potassium thiocyanate (KSCN) solution at −4 °C. The dissolution behavior and the mechanism of the dissolution of deacetylated KGM (da-KGM) were studied in detail. The results from differential scanning calorimetry (DSC), Fourier transform infrared spectroscopy (FTIR) and viscometry demonstrated the existence of KSCN hydrate at low temperatures. With increasing KSCN concentration, the number of bound water molecules per KSCN molecule decreased. Low temperature aided the disintegration of da-KGM molecular chains, prevented the approach of da-KGM molecules toward each other and prompted the binding of da-KGM and KSCN hydrate to form a larger complex by hydrogen bonding. The result of CP-MAS 13C NMR spectroscopy indicated that KSCN could be a non-derivative solvent of da-KGM since neither obvious changes in molecular chain structure occurred, nor new derivatives generated after the dissolution of da-KGM.
Introduction
Konjac Glucomannan (KGM) is a neutral polysaccharide derived from the tuber of Amorphophallus konjac and native to Southeast Asia. It is composed of β-(1,4)-linked D-mannose and D-glucose residues in a molar ratio of 1.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.1,2 Acetyl groups attached to the saccharide units at C-6 positions are scattered randomly along the molecule with an occurrence of approximately 1 per 19 sugar residues.3 Acetyl groups can control the water-solubility of KGM fractions.4 The acetylated KGM fractions can be thoroughly hydrated to form homogeneous aqueous dispersions in relatively shorter time compared with native KGM. The hydration time was gradually reduced with an increasing degree of acetylation.5 Meanwhile, the removal of acetyl groups led to poor water solubility of KGM.6 It was very difficult to dissolve deacetylated KGM (da-KGM) in aqueous solutions due to considerable inter- and/or intra-molecular hydrogen bonds.
1.1,2 Acetyl groups attached to the saccharide units at C-6 positions are scattered randomly along the molecule with an occurrence of approximately 1 per 19 sugar residues.3 Acetyl groups can control the water-solubility of KGM fractions.4 The acetylated KGM fractions can be thoroughly hydrated to form homogeneous aqueous dispersions in relatively shorter time compared with native KGM. The hydration time was gradually reduced with an increasing degree of acetylation.5 Meanwhile, the removal of acetyl groups led to poor water solubility of KGM.6 It was very difficult to dissolve deacetylated KGM (da-KGM) in aqueous solutions due to considerable inter- and/or intra-molecular hydrogen bonds.
Till now, da-KGM has been proved to be a good fat analogue.7 Interestingly, deacetylation and freezing could significantly improve the gel strength and even resulted in fibrosis.8 Thus da-KGM, as a material, is expected to have numerous applications in frozen processing such as fiber modification, dissolution and regeneration. It was reported in 1973 that the peptization of KGM gel was easier when carried out at lower temperature.9 Perols embedded enzyme into konjac gel and released the enzyme at 4 °C.10 Low temperature was conducive to the dissolution of da-KGM.11 Other dissolution techniques of da-KGM, including using organic solvent and ionic liquid, have not been reported. Therefore, the dissolution of da-KGM posed scientific and technical challenges.
With respect to the dissolution of cellulose, researchers have done a lot of work.12 In 1939, Sobue published that at temperatures of −5 to −10 °C, NaOH could dissolve cellulose.13 The aqueous solution of NaOH–urea–H2O (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12
12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 81 by weight) that was precooled to −10 °C, had been successfully developed to completely dissolve cellulose within 5 min at ambient temperature.14 As solvents of cellulose, some salts, such as LiCl/DMAc,15 Ca(SCN)2/H2O,16 inorganic molten salts17 and NH3/NH4SCN, have made great contribution.18,19 Both the type and the concentration of salt played important roles in cellulose dissolution. Recently, Frey investigated the dissolution of cellulose in ethylene diamine/salt solvent systems,17,18,20,21 and found that among the solvents NaSCN, KSCN or KI, KSCN was capable of dissolving both high molecular weight (degree of polymerization DP > 1000) and low molecular weight (DP = 210) cellulose.22 The K+ ion interacted with cellobiose more than the SCN− ion. A possible dissolution mechanism was that the KSCN was able to diffuse into highly crystalline regions of cellulose and interacted with the hydroxyl groups of cellulose, which led to the breaking of hydrogen bonds and development of new hydrogen bonds at new positions within the cellulose chains.23
81 by weight) that was precooled to −10 °C, had been successfully developed to completely dissolve cellulose within 5 min at ambient temperature.14 As solvents of cellulose, some salts, such as LiCl/DMAc,15 Ca(SCN)2/H2O,16 inorganic molten salts17 and NH3/NH4SCN, have made great contribution.18,19 Both the type and the concentration of salt played important roles in cellulose dissolution. Recently, Frey investigated the dissolution of cellulose in ethylene diamine/salt solvent systems,17,18,20,21 and found that among the solvents NaSCN, KSCN or KI, KSCN was capable of dissolving both high molecular weight (degree of polymerization DP > 1000) and low molecular weight (DP = 210) cellulose.22 The K+ ion interacted with cellobiose more than the SCN− ion. A possible dissolution mechanism was that the KSCN was able to diffuse into highly crystalline regions of cellulose and interacted with the hydroxyl groups of cellulose, which led to the breaking of hydrogen bonds and development of new hydrogen bonds at new positions within the cellulose chains.23
However, the dissolution behavior and solubility of da-KGM has not been explored yet. This is the first study to investigate the dissolution of da-KGM in aqueous KSCN solutions at −4 °C. The dissolution mechanism was studied in detail by CP-MAS 13C NMR, FT-IR, DSC and viscometry. A fundamental understanding of dissolution mechanisms might help in the search for more and better solvents for da-KGM, broaden the application of KGM in new fields and provide information regarding the dissolution of other native polysaccharides.
Materials and methods
Materials
Native KGM sample was purchased from Hubei Jian Shi Nong Tai Industry Co. Ltd. (Hubei, China). All chemicals were of analytical grade (Sinopharm Chemical Reagent Co. Ltd., Shanghai, China) and were used without further purification.
da-KGM preparation
KGM with different degree of deacetylation (DD) were prepared according to a previously reported method.24 In brief, 30 g KGM powder was mixed with 50 mL 75% ethanol, which was kept and stirred in a constant thermostat oscillator at 40 °C and 150 rpm min−1 for 30 min. Then, a certain amount of Na2CO3 solution was added and the mixture was reacted for 24 h. After deacetylation, the sample was successively washed three times with 50%, 75% and 95% ethanol to remove excess alkali before washing with absolute ethanol. The excess ethanol was evaporated in a fume cupboard followed by vacuum drying for 6 h at 40 °C and the powdered da-KGM was obtained. DD of KGM was controlled by changing the amount of Na2CO3, and the obtained da-KGM was coded as D1, D3 and D5 with DD at 20%, 40% and 80%, respectively.25
Powdered da-KGM was dispersed in aqueous KSCN solution at −4 °C, and then stirred for 1 h to obtain a homogeneous solution. After being dialyzed against distilled water and lyophilized, a regenerated da-KGM was obtained for analysis.
Solubility measurement
da-KGM with different DD was immediately added into 9 wt% KSCN solution with vigorous stirring for 1 h at −4 °C. The dispersed solution was then centrifuged at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm and 4 °C for 20 min. The remaining undissolved fractions were then washed with distilled water, and dried at 105 °C to a constant weight in a vacuum oven. The dissolution degree S was defined by the equation:
000 rpm and 4 °C for 20 min. The remaining undissolved fractions were then washed with distilled water, and dried at 105 °C to a constant weight in a vacuum oven. The dissolution degree S was defined by the equation:| | |
S = [(W0 − Wr)/W0] × 100%
| (1) |
where Wr was the weight of undissolved da-KGM residue and W0 was the original weight of da-KGM.
Characterization
DSC measurements were performed on a Netzsch DSC-204-F1 calorimeter (Netzsch Co., Germany). 17 ± 0.1 mg of the solution in an aluminum crucible with a reference sample in an empty crucible were analyzed in a dried N2 gas atmosphere at a heating rate of 10 °C min−1 in a temperature range of −60 °C to 30 °C. Sweep gas rate was 20 mL min−1 and protective gas rate was 60 mL min−1. Pure aqueous KSCN solutions ranged from 6 wt% to 40 wt% (denoted KS6, KS9, etc.). da-KGM (D5) solutions ranging from 0 wt% to 1.5 wt% dissolved in KSCN solutions were used.
The zero-shear viscosity of the solution was determined using the steady shear tests on an AR2000ex rheometer (TA Instruments Ltd., Gloucestershire, UK). Double-concentric cylinder geometry with a gap of 2 mm was used to measure shear viscosity as a function of shear rate. Temperature control was established by connecting with a cooling/heating bath maintained within 0.2 °C over an extended time.
FTIR spectra of H2O and aqueous KSCN solution were recorded on a spectrometer (a Bruker Equonox-55S, Germany) equipped with a variable-temperature cell (P/N 21525, SPECA Inc.). For each spectrum, 32 scans were recorded and averaged. Low temperature measurements were performed with a laboratory-designed liquid-nitrogen-cooled cryostat consisting of a copper sample holder with a small container that could be filled with liquid nitrogen. This setup was surrounded by a jacket with KBr windows and placed under N2 gas flow. The solvent was sealed between the two CaF2 crystals. Data were obtained at temperatures ranging from −5 °C to 15 °C.
Solid-state 13C NMR spectra were recorded on an Infinity Plus 400 spectrometer (Varian, magnetic field: 9.4 T, 13C frequency: 100.12 MHz) with a CP-MAS unit at room temperature. The spinning rate and contact time were 5.0 kHz and 5.0 ms, respectively. The pulse width was 2.10 μs, the spectral width was 50.0 kHz, the acquisition time was 20.48 ms and the spectrum was accumulated 2000 times.
Results and discussion
Solubility of da-KGM in KSCN solution
The solubility of da-KGM with different degrees of deacetylation is presented in Fig. 1A. The solubility of three samples increased rapidly and reached almost 100% in the first 60 min. It revealed that KSCN solution was able to dissolve da-KGM at −4 °C. In contrast, da-KGM could partly dissolve in ice-water (0 °C) under vigorous stirring.6
 |
| | Fig. 1 (A) Concentration of da-KGM in 9 wt% KSCN solution as a function of time; (B) plot of the fitting kinetics of da-KGM in 9 wt% KSCN solution. | |
The process of dissolution could be treated as a pseudo-first-order reaction. The dissolution rate constant k was obtained based on the equation: −ln(1 − S) = kt. The fitting and coefficients are shown in Fig. 1B. The k values of D1, D3 and D5 were 0.057, 0.051 and 0.044 min−1, respectively, suggesting that a lower DD could accelerate the dissolution of da-KGM. The activation enthalpy (ΔH) was derived by Eyring equation:26,27
| |
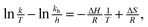 | (2) |
where
T is the absolute temperature,
kb is the Boltzmann constant,
h is the Planck's constant,
R is the gas constant and Δ
S is entropy activation. Δ
H values of D1, D3and D5 were calculated to be −6.41, −6.66, −7.00 kJ mol
−1, respectively. It was documented that when Δ
H < 0, the reaction was exothermic, favoring lower temperature.
28 In Ramos' work, cellulose was dissolved in a LiCl/DMAc solvent and the decrystallization enthalpy was −7.62 kJ mol
−1.
29 Wang dissolved cellulose in NaOH–urea solution and obtained an enthalpy of −61.82 kJ mol
−1.
30
KSCN molecular structure in aqueous solution
To better understand the dissolution of da-KGM in KSCN solution, the dissolution mechanism was explored. The special KSCN structure in aqueous solution was first examined at low temperature (Fig. 2). As shown in Fig. 2A, there were two endothermic peaks, one above 0 °C and one below −20 °C, which were assigned to the melting peak of free water and KSCN hydrate in KSCN aqueous solution, respectively. With increasing KSCN concentration, the melting peak of free water shifted to lower temperature and became smaller, while that of KSCN hydrate occurred at the same temperature but with higher intensity. Interestingly, there was only one peak corresponding to the melting peak of KSCN hydrate in KS40 (40 wt% KSCN solution), suggesting that free water did not exist when the concentration of KSCN was higher than 40 wt%. The fraction of free (Ffree) and bound water (Fbound) in KSCN hydrate were defined as follows. The number of bound water molecules per KSCN molecule (X) was expressed as a function of KSCN concentration (CKSCN).| |
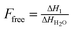 | (3) |
| |
 | (5) |
where Ffree was calculated as a ratio of the melting enthalpy (ΔH1) of the first peak to that of the pure water (ΔHH2O = 332 J g−1); NKSCN and NH2O were the number of KSCN and water molecules in KSCN hydrate, respectively; MKSCN (97 g mol−1) and MH2O (18 g mol−1) were the molar masses of KSCN and water. The calculated Fbound and X in KSCN hydrate were summarized in Table 1. The number fraction (Fbound) increased and number (X) decreased with higher KSCN concentration. In addition, a certain specific enthalpy (ΔHsp) corresponding only to bound-to-KSCN water was independent on KSCN concentration and should be ΔH2 = 242 J g−1 (pure KSCN hydrate at 40 wt% KSCN solution). Such a ΔHsp value for each KSCN concentration could be calculated assuming that the KSCN hydrate consisted of eight bound H2O molecules (X = 8) and amorphous water molecules by eqn (6):| |
 | (6) |
where ΔHsp was the experimental value of the second peak at a given KSCN concentration, msample was the total mass of sample and mKSCN·(H2O)8 was the mass of the KSCN·(H2O)8 hydrate. The latter could be calculated by eqn (7):| |
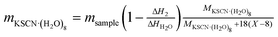 | (7) |
where ΔH2 was the enthalpy value of the low-temperature peak at the desired KSCN concentration, ΔHH2O (332 J g−1) was the enthalpy of water, MKSCN·(H2O)8 (241 g mol−1) was the molar mass of KSCN·(H2O)8 and MKSCN·(H2O)8 + 18(X − 8) was the molar mass of total KSCN hydrate with bound and amorphous water. The ΔHsp value for each KSCN concentration was calculated to be 226–255 J g−1, which was close to the melting enthalpy of the peak at KS40 (242 J g−1). It could be speculated that KSCN hydrate consisted of a ratio of around 1/8 for the molecular number ratio of KSCN and water, thereby indicating the existence of the KSCN·(H2O)8 hydrate cluster. Cai similarly demonstrated the existence of the urea·(H2O)10 hydrate.31 Roy proposed a soda + water structural model in the 0–20% NaOH that consisted of three phases: free water, NaOH hydrate, composed of nine water molecules per NaOH molecule, and a shell of uncrystallizable water around the hydrate.32
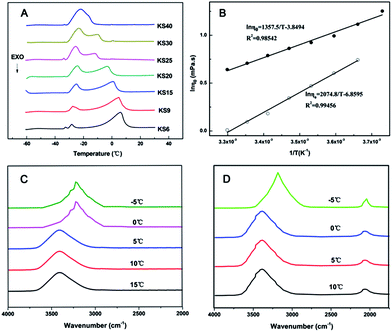 |
| | Fig. 2 Characterization of KSCN hydrate. (A) DSC thermograms of aqueous KSCN solution at concentrations ranging from 6 wt% to 40 wt% (denoted KS6, KS9, KS15, KS20, KS25, KS30 and KS40); (B) Arrhenius plots of zero-shear viscosity of pure 9 wt% KSCN (●) solution and H2O (○); (C) FT-IR spectra of H2O at different temperatures; (D) FT-IR spectra of KSCN at different temperatures. | |
Table 1 Experimental results of the number fraction (Fbound) of bound water and the number (X) of bound water molecules per KSCN molecule in aqueous KSCN solutions
| CKSCN (wt%) |
Fbound |
X |
| 6 |
0.24 ± 0.02 |
20 |
| 9 |
0.30 ± 0.02 |
16 |
| 15 |
0.50 ± 0.02 |
15 |
| 20 |
0.60 ± 0.02 |
13 |
| 25 |
0.76 ± 0.02 |
12 |
| 30 |
0.87 ± 0.02 |
11 |
| 40 |
1.0 ± 0.02 |
8 |
| 50 |
1.0 ± 0.02 |
5 |
Interaction between KSCN and H2O molecules
To further investigate the temperature effect and gain a more detailed insight into KSCN solution, the logarithm of zero-shear viscosity was plotted against the reciprocal temperature (Fig. 2B). The fitting equations and coefficients are given in the inset of Fig. 2B. A good linear fit indicated that the zero-shear viscosity of KSCN solution exhibited an Arrhenius behavior. At low temperature, the original structure of water molecules was destroyed, accompanying the reorganization of hydrogen bonds to form larger structures, which eventually caused the slow movement of water molecules and the increase of the viscosity.33,34 Zero shear viscosity of 9 wt% KSCN solution also showed an upward trend as the temperature decreased. Therefore, the interaction between KSCN and H2O and the formation of KSCN hydrate at low temperature could be confirmed.
FTIR spectra of H2O and aqueous KSCN solution at various temperatures are shown in Fig. 2C and D. The peaks observed at about 3400 cm−1 and 2100 cm−1 were attributable to the stretching of –OH and –SCN, respectively.21 As shown in Fig. 2C, the peak of –OH did not change with temperature above 0 °C. When the temperature was approaching 0 °C and even lower, the band shifted to blue and its intensity increased, suggesting that the damage of the original tetrahedral structure and hydrogen bonding reorganization of water molecules occurred at low temperature.25,29,31,35 Meanwhile, the peak of –OH in aqueous KSCN solution was similar to that of pure water. As the temperature decreased to −5 °C, the peak intensity of –SCN also increased. It indicated that hydrogen bond formation between KSCN and water in the aqueous mixture at lower temperature could occur more easily than that at higher temperatures.
Interaction between KSCN and da-KGM molecules
Based on the abovementioned results, the interaction of KSCN and da-KGM molecules was subsequently investigated. Table 2 presents the change in melting temperatures and enthalpies of da-KGM in KSCN solution and pure KSCN solution as a function of KSCN concentration. Both temperature and enthalpy were similar and decreased with increasing KSCN concentration. Therefore, there was no effect of da-KGM on the melting temperature and enthalpy of free water. This was because the dispersion of da-KGM molecules in solution could only lead to an increased amount of solute, and could be located in KSCN hydrate regions. The presence of da-KGM did not change the amount of free water in KSCN solutions but changed the structure of KSCN hydrate.
Table 2 Melting temperatures and enthalpies of da-KGM + KSCN + H2O (Cda-KGM = 1%) and KSCN + H2O solutions for higher temperature peak as a function of KSCN concentration
| CKSCN (wt%) |
da-KGM + KSCN + H2O |
KSCN + H2O |
| T1 (°C) |
ΔH1 (J g−1) |
T1 (°C) |
ΔH1 (J g−1) |
| 6 |
6.8 |
276.4 |
8.7 |
254.4 |
| 9 |
0.9 |
251 |
6.5 |
231.8 |
| 15 |
−2.1 |
171.2 |
1.5 |
164.1 |
| 20 |
−3.6 |
148.5 |
−2.7 |
130.2 |
| 25 |
−11.3 |
108.5 |
−10.5 |
80.2 |
| 30 |
−11.8 |
58.7 |
−11.2 |
40.28 |
| 40 |
−20.4 |
0 |
−20.4 |
0 |
The influence of da-KGM on its thermal properties in KSCN solution was detected by varying da-KGM concentration and maintaining a constant KSCN concentration at 9 wt%. The melting temperatures and enthalpies of hydrate peak decreased with an increase in da-KGM concentration (Table 3). It implied that the number of free KSCN hydrate reduced. A certain fraction of hydrate KSCN and da-KGM were bound to form a larger complex, which prevented the association between KGM molecules. The dissolution could be described as a heterogeneous reaction involving the disintegration of molecular chains, the solvation of molecules and the diffusion of the solvated polymer into the solvent.36
Table 3 Melting temperatures and enthalpies of da-KGM + KSCN + H2O solutions as a function of da-KGM concentration, CKSCN = 9%
| Cda-KGM (wt%) |
High temperature peak (ice melting) |
Low temperature peak (hydrate melting) |
| T1 (°C) |
ΔH1 (J g−1) |
T2 (°C) |
ΔH2 (J g−1) |
| 0 |
8.5 |
231.8 |
−24.1 |
42.21 |
| 0.8 |
1.4 |
245.8 |
−27.8 |
38.25 |
| 1.0 |
0.8 |
251 |
−30.1 |
34.47 |
| 1.5 |
−1.9 |
249.4 |
−32.7 |
27.36 |
Using the values of enthalpies obtained for the melting peaks (Table 3), the fraction of free KSCN hydrates (F) and bound-to-da-KGM KSCN hydrates (B) could be calculated as follows:
| |
 | (8) |
where
mfree was the mass of free KSCN hydrates and
mhydrate was the mass of all KSCN hydrates in da-KGM with KSCN solution. It was observed that eight H
2O molecules were bound to KSCN and eight amorphous water molecules when the concentration of KSCN solution was 9 wt% (
Table 1). We assumed that the number of water molecules in the amorphous shell was the same in da-KGM with KSCN solution and pure KSCN solutions. The mass of free KSCN hydrate
mfree could be calculated from the hydrate-specific enthalpy value obtained in the previous equation:
| |
 | (10) |
where
msample was the mass of the total sample, Δ
H2(da-KGM) was the enthalpy of the second peak corresponding to KSCN hydrates in da-KGM + KSCN + H
2O solution and Δ
Hsp was the specific enthalpy of KSCN·(H
2O)
8 (242 J g
−1). The mass of all KSCN·(H
2O)
8 in da-KGM with KSCN solution could be calculated by the following equation:
| |
 | (11) |
| |
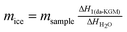 | (12) |
where
mice was the mass of free water in da-KGM + KSCN + H
2O solution, which was calculated as a ratio between the enthalpy of the free water peak Δ
H1(da-KGM) at a corresponding da-KGM concentration and the enthalpy of pure water Δ
HH2O (332 J g
−1). The result obtained for the fraction of free and bound-to-da-KGM KSCN hydrate (B) is presented in
Table 4. The number of bound-to-da-KGM increased with increasing da-KGM concentration, which illustrated that KSCN promoted the dissolution of da-KGM by destroying the hydrogen bonds among da-KGM molecules. A similar phenomenon was also observed in cellulose when it was dissolved in 7 wt% NaOH–12 wt% urea aqueous solution pre-cooled to −12 °C, inclusion complexes were formed associated with cellulose, urea and NaOH.
37
Table 4 Fraction of bound-to-da-KGM KSCN hydrates and free KSCN hydrates in da-KGM/9 wt% KSCN solution
| Cda-KGM (wt%) |
B (%) |
F (%) |
| 0.8 |
35 |
65 |
| 1.0 |
48 |
52 |
| 1.5 |
50 |
50 |
KGM structure of da-KGM before and after dissolution
The solid state 13C NMR spectra of da-KGM (D5) and regenerated one (RD5) were shown in Fig. 3 and the resonances of carbon atoms in KGM molecules were obvious.38,39 The signals of the carbon atoms C1–C6 were well resolved. Compared with the peaks of D5, peaks of RD5 at 102.9 ppm and 74.8 ppm were narrower and higher, which implied that regenerated da-KGM had a more orderly structure after dissolution in 9 wt% KSCN solution. Cuculo et al. pointed out that crystallinity had an effect on the 13CNMR spectra of cellulose. Lower crystallinity led to broadening of peaks, while high crystallinity led to narrowing of peaks.18 In addition, the splitting of C-6 peaks in RD5 occurred. This was very likely related to the breaking of inter-hydrogen bonds of da-KGM and the formation of the intermolecular hydrogen bonds between da-KGM and KSCN hydrate. Furthermore, a new peak appeared at 18.4 ppm in RD5, which belonged to the chemical shift of C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N.22,40 This revealed that KSCN was not completely removed by washing. Overall, there were no new peaks for da-KGM derivates in the 13C NMR spectra, indicating the absence of derivatization. Namely, there was no chemical reaction in this system, and KSCN was a direct solvent of da-KGM. Moulthrop et al. analyzed the 13C NMR spectra for cellulose and cellulose oligomers and stated that the ionic liquids were true nonderivatizing/nondegrading cellulose solvents.41
N.22,40 This revealed that KSCN was not completely removed by washing. Overall, there were no new peaks for da-KGM derivates in the 13C NMR spectra, indicating the absence of derivatization. Namely, there was no chemical reaction in this system, and KSCN was a direct solvent of da-KGM. Moulthrop et al. analyzed the 13C NMR spectra for cellulose and cellulose oligomers and stated that the ionic liquids were true nonderivatizing/nondegrading cellulose solvents.41
 |
| | Fig. 3 Solid state CP-MAS 13C NMR spectra of da-KGM and regenerated da-KGM. | |
The dissolution mechanism of da-KGM in aqueous KGM solutions at low temperature (−4 °C) was proposed based on the abovementioned results (Fig. 4). When da-KGM was immersed in the solvent, its molecules were surrounded by KSCN and free water molecules (shown in Fig. 4a). At low temperature, KSCN hydrates and free water penetrated the da-KGM molecules and destroyed the intra- and inter-molecular hydrogen bonding, resulting in the solvation of the da-KGM chains (Fig. 4b). At this stage, da-KGM imbibed the hydrates and expanded to a swollen gel. Fig. 4c shows the da-KGM chains, which were surrounded with KSCN hydrates and free water as an overcoat, dispersed in the aqueous solution to form a transparent solution.
 |
| | Fig. 4 Schematic dissolution process of da-KGM in 9 wt% KSCN solution at 4 °C. | |
Conclusions
At room temperature, da-KGM was insoluble in water because of considerable inter- and/or intra-molecular hydrogen bonds. When immersed in aqueous KSCN solutions at −4 °C, the solubility of KGM could reach 100%, which could not be affected by DD of KGM. DSC, FT-IR and viscosity measurement results proved the existence of the KSCN·(H2O)8 hydrate cluster. The number of amorphous water molecules bound to the KSCN·(H2O)8 was related to KSCN concentration. During the dissolution of da-KGM, its molecules could combine with a certain fraction of KSCN hydrates, resulting in the formation of a new larger complex. The fraction of water bound-to-da-KGM was higher with increasing da-KGM concentration. KSCN hydrates could prevent the approach of the da-KGM molecules toward each other, leading to the good dispersion of da-KGM to give an actual solution. 13C NMR showed that the molecular structure of da-KGM became more orderly after it was dissolved in aqueous KSCN solutions. KSCN could be a non-derivative solvent of da-KGM as there was no change in KGM molecular chain structure, and new derivatives were generated after the dissolution of da-KGM. The dissolution could be deduced as a heterogeneous reaction involving the disintegration of molecular chains, the solvation of molecules and the diffusion of the solvated polymer into the solvent. The solvent system at low temperature would be important for further developments in the dissolution of native polysaccharides.
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (Grant no. 31371841).
Notes and references
- P. Cescutti, C. Campa, F. Delben and R. Rizzo, Carbohydr. Res., 2002, 337, 2505–2511 CrossRef CAS.
- Q. Xu, W. Huang, L. Jiang, Z. Lei, X. Li and H. Deng, Carbohydr. Polym., 2013, 97, 565–570 CrossRef CAS PubMed.
- K. Katsuraya, K. Okuyama, K. Hatanaka, R. Oshima, T. Sato and K. Matsuzaki, Carbohydr. Polym., 2003, 53, 183–189 CrossRef CAS.
- J. Hwang and J. Kokini, J. Texture Stud., 1991, 22, 123–167 CrossRef CAS.
- S. Gao and K. Nishinari, Biomacromolecules, 2004, 5, 175–185 CrossRef CAS PubMed.
- X. Du, J. Li, J. Chen and B. Li, Food Res. Int., 2012, 46, 270–278 CrossRef CAS PubMed.
- B. Herranz, C. A. Tovar, B. Solo-de-Zaldivar and A. Javier Borderias, Food Hydrocolloids, 2012, 27, 145–153 CrossRef CAS PubMed.
- B. Li and D.-W. Sun, J. Food Eng., 2002, 54, 175–182 CrossRef.
- Maekaji, Agric. Biol. Chem., 1973, 37, 2433–2434 CrossRef CAS.
- C. Perols, B. Piffaut, J. Scher, J. Ramet and D. Poncelet, Enzyme Microb. Technol., 1997, 20, 57–60 CrossRef CAS.
- S. Wang, Y. Zhan, X. Wu, T. Ye, Y. Li, L. Wang, Y. Chen and B. Li, Carbohydr. Polym., 2014, 101, 499–504 CrossRef CAS PubMed.
- H. Deng, X. Zhou, X. Wang, C. Zhang, B. Ding, Q. Zhang and Y. Du, Carbohydr. Polym., 2010, 80, 474–479 CrossRef CAS PubMed.
- H. Sobue, H. Kiessig and K. Hess, Zeits-chrift Fur Physikalische Chemie-Abteilung B-Chemie Der Elementarprozesse Aufbau Der Material, 1939, 43, 309–328 Search PubMed.
- J. Cai, L. Zhang, J. Zhou, H. Li, H. Chen and H. Jin, Macromol. Rapid Commun., 2004, 25, 1558–1562 CrossRef CAS.
- A. M. Striegel, J. Chil. Chem. Soc., 2003, 48, 73–77 CAS.
- M. Hattori, T. Koga, Y. Shimaya and M. Saito, Polym. J., 1998, 30, 43–48 CrossRef CAS.
- S. Fischer, H. Leipner, K. Thümmler, E. Brendler and J. Peters, Cellulose, 2003, 10, 227–236 CrossRef CAS.
- J. Cuculo, C. Smith, U. Sangwatanaroj, E. Stejskal and S. Sankar, J. Polym. Sci., Part A: Polym. Chem., 1994, 32, 229–239 CrossRef CAS.
- J. Cuculo, C. Smith, U. Sangwatanaroj, E. Stejskal and S. Sankar, J. Polym. Sci., Part A: Polym. Chem., 1994, 32, 241–247 CrossRef CAS.
- M. W. Frey, H. Chan and K. Carranco, J. Polym. Sci., Part B: Polym. Phys., 2005, 43, 2013–2022 CrossRef CAS.
- M. W. Frey, L. Li, M. Xiao and T. Gould, Cellulose, 2006, 13, 147–155 CrossRef CAS.
- M. Xiao and M. W. Frey, Cellulose, 2009, 16, 381–391 CrossRef CAS PubMed.
- M. Xiao and M. W. Frey, Cellulose, 2007, 14, 225–234 CrossRef CAS PubMed.
- J. Chen, J. Li and B. Li, Carbohydr.
Polym., 2011, 86, 865–871 CrossRef CAS PubMed.
- L. J. Tanghe, L. B. Genung and J. W. Mench, Methods Carbohydr. Chem., 1963, 3, 201–203 CAS.
- Y. Ma, S. Meng, M. Qin, H. Liu and Y. Wei, J. Phys. Chem. Solids, 2012, 73, 30–34 CrossRef CAS PubMed.
- O. Ozay, N. Aktas, E. Inger and N. Sahiner, Int. J. Hydrogen Energy, 2011, 36, 1998–2006 CrossRef CAS PubMed.
- A. Sullo, Y. Wang, J. Mitchell, A. Koshella, T. Heinze and T. Foster, Gums and Stabilisers for the Food Industry 16, 2012, 16, 45 Search PubMed.
- L. A. Ramos, J. M. Assaf, O. A. El Seoud and E. Frollini, Biomacromolecules, 2005, 6, 2638–2647 CrossRef CAS PubMed.
- Y. Wang and Y. Deng, Biotechnol. Bioeng., 2009, 102, 1398–1405 CrossRef CAS PubMed.
- J. Cai, L. Zhang, C. Chang, G. Cheng, X. Chen and B. Chu, ChemPhysChem, 2007, 8, 1572–1579 CrossRef CAS PubMed.
- C. Roy, T. Budtova, P. Navard and O. Bedue, Biomacromolecules, 2001, 2, 687–693 CrossRef CAS.
- H. Yu and W. F. van Gunsteren, J. Chem. Phys., 2004, 121, 9549 CrossRef CAS PubMed.
- R. Ludwig, Angew. Chem., Int. Ed., 2001, 40, 1808–1827 CrossRef CAS.
- J.-B. Brubach, A. Mermet, A. Filabozzi, A. Gerschel and P. Roy, J. Chem. Phys., 2005, 122, 184509 CrossRef PubMed.
- H. Qi, C. Chang and L. Zhang, Cellulose, 2008, 15, 779–787 CrossRef CAS.
- X. Qin, A. Lu, J. Cai and L. Zhang, Carbohydr. Polym., 2013, 92, 1315–1320 CrossRef CAS PubMed.
- M. Vieira and A. Gil, Carbohydr. Polym., 2005, 60, 439–448 CrossRef CAS PubMed.
- M. Gidley, A. McArthur and D. Underwood, Food Hydrocolloids, 1991, 5, 129–140 CrossRef CAS.
- J. L. Jane, Starch/Staerke, 1993, 45, 172–175 CrossRef CAS.
- J. S. Moulthrop, R. P. Swatloski, G. Moyna and R. D. Rogers, Chem. Commun., 2005, 1557–1559 RSC.
|
| This journal is © The Royal Society of Chemistry 2014 |
Click here to see how this site uses Cookies. View our privacy policy here. ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.1,2 Acetyl groups attached to the saccharide units at C-6 positions are scattered randomly along the molecule with an occurrence of approximately 1 per 19 sugar residues.3 Acetyl groups can control the water-solubility of KGM fractions.4 The acetylated KGM fractions can be thoroughly hydrated to form homogeneous aqueous dispersions in relatively shorter time compared with native KGM. The hydration time was gradually reduced with an increasing degree of acetylation.5 Meanwhile, the removal of acetyl groups led to poor water solubility of KGM.6 It was very difficult to dissolve deacetylated KGM (da-KGM) in aqueous solutions due to considerable inter- and/or intra-molecular hydrogen bonds.
1.1,2 Acetyl groups attached to the saccharide units at C-6 positions are scattered randomly along the molecule with an occurrence of approximately 1 per 19 sugar residues.3 Acetyl groups can control the water-solubility of KGM fractions.4 The acetylated KGM fractions can be thoroughly hydrated to form homogeneous aqueous dispersions in relatively shorter time compared with native KGM. The hydration time was gradually reduced with an increasing degree of acetylation.5 Meanwhile, the removal of acetyl groups led to poor water solubility of KGM.6 It was very difficult to dissolve deacetylated KGM (da-KGM) in aqueous solutions due to considerable inter- and/or intra-molecular hydrogen bonds.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12
12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 81 by weight) that was precooled to −10 °C, had been successfully developed to completely dissolve cellulose within 5 min at ambient temperature.14 As solvents of cellulose, some salts, such as LiCl/DMAc,15 Ca(SCN)2/H2O,16 inorganic molten salts17 and NH3/NH4SCN, have made great contribution.18,19 Both the type and the concentration of salt played important roles in cellulose dissolution. Recently, Frey investigated the dissolution of cellulose in ethylene diamine/salt solvent systems,17,18,20,21 and found that among the solvents NaSCN, KSCN or KI, KSCN was capable of dissolving both high molecular weight (degree of polymerization DP > 1000) and low molecular weight (DP = 210) cellulose.22 The K+ ion interacted with cellobiose more than the SCN− ion. A possible dissolution mechanism was that the KSCN was able to diffuse into highly crystalline regions of cellulose and interacted with the hydroxyl groups of cellulose, which led to the breaking of hydrogen bonds and development of new hydrogen bonds at new positions within the cellulose chains.23
81 by weight) that was precooled to −10 °C, had been successfully developed to completely dissolve cellulose within 5 min at ambient temperature.14 As solvents of cellulose, some salts, such as LiCl/DMAc,15 Ca(SCN)2/H2O,16 inorganic molten salts17 and NH3/NH4SCN, have made great contribution.18,19 Both the type and the concentration of salt played important roles in cellulose dissolution. Recently, Frey investigated the dissolution of cellulose in ethylene diamine/salt solvent systems,17,18,20,21 and found that among the solvents NaSCN, KSCN or KI, KSCN was capable of dissolving both high molecular weight (degree of polymerization DP > 1000) and low molecular weight (DP = 210) cellulose.22 The K+ ion interacted with cellobiose more than the SCN− ion. A possible dissolution mechanism was that the KSCN was able to diffuse into highly crystalline regions of cellulose and interacted with the hydroxyl groups of cellulose, which led to the breaking of hydrogen bonds and development of new hydrogen bonds at new positions within the cellulose chains.23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm and 4 °C for 20 min. The remaining undissolved fractions were then washed with distilled water, and dried at 105 °C to a constant weight in a vacuum oven. The dissolution degree S was defined by the equation:
000 rpm and 4 °C for 20 min. The remaining undissolved fractions were then washed with distilled water, and dried at 105 °C to a constant weight in a vacuum oven. The dissolution degree S was defined by the equation:
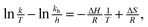
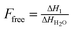


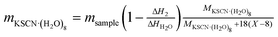



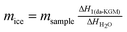
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N.22,40 This revealed that KSCN was not completely removed by washing. Overall, there were no new peaks for da-KGM derivates in the 13C NMR spectra, indicating the absence of derivatization. Namely, there was no chemical reaction in this system, and KSCN was a direct solvent of da-KGM. Moulthrop et al. analyzed the 13C NMR spectra for cellulose and cellulose oligomers and stated that the ionic liquids were true nonderivatizing/nondegrading cellulose solvents.41
N.22,40 This revealed that KSCN was not completely removed by washing. Overall, there were no new peaks for da-KGM derivates in the 13C NMR spectra, indicating the absence of derivatization. Namely, there was no chemical reaction in this system, and KSCN was a direct solvent of da-KGM. Moulthrop et al. analyzed the 13C NMR spectra for cellulose and cellulose oligomers and stated that the ionic liquids were true nonderivatizing/nondegrading cellulose solvents.41



