Degradation of phenol by air and polyoxometalate nanofibers using a continuous mode†
Jian Xua,
Siqi Yana,
Jianxin Lia,
Shengtian Wanga,
Xiaohong Wang*a,
Mingxin Huo*b and
Zijiang Jiang*c
aKey Lab of Polyoxometalate Science of Ministry of Education, Faculty of Chemistry, Northeast Normal University, 5268 Renmin Street, Changchun 130024, P. R. China. E-mail: wangxh665@nenu.edu.cn; Fax: +86-431-85099759; Tel: +86-431-88930042
bSchool of Urban and Environmental Sciences, Northeast Normal University, Changchun 130024, P. R. China
cChangchun Institute of Applied Chemistry, Chinese Academy of Sciences, Changchun 130024, P. R. China
First published on 13th May 2014
Abstract
Nanofibers were synthesized by an electrospinning technique using a polymer as a support and polyoxometalates (POMs) as dopants. X-ray diffraction (XRD), Fourier transform infrared (FT-IR) spectroscopy, 31P MAS NMR, scanning electron microscopy (SEM), and a high resolution transmission electron microscope (TEM) were used to characterize the resulting hybrids. These POM nanofiber catalysts could provide a continuous flowing mode to promote the oxidative degradation of phenol into simpler inorganic species using air (O2) as an oxidant at room temperature through nine cycles. The leaching of POMs from the nanofibers was minor, showing that the catalyst had excellent stability and could be used as a heterogeneous structure.
1. Introduction
Phenol is an important industrial chemical because of its wide usage (e.g., as a disinfectant, a precursor of phenolic resins, and a reagent in chemical synthesis); however, it is a very serious pollutant because of its high stability, low biodegradability,1 and toxicity at low concentrations.2 Many techniques based on both chemical conversions and physical operations have been used to remove phenol from wastewater such as photocatalytic degradation,3 ultrasound-assisted degradation,4 catalytic wet air oxidation (CWAO),5,6 and microwave-enhanced catalytic degradation.7,8In the CWAO process, many catalysts were involved, including both homogeneous and heterogeneous transition-metal catalysts (Cu2+, Fe3+, or Mn2+)9–12 and polyoxometalates.13,14 POMs are available for the CWAO of phenol due to their outstanding redox behavior and unique Keggin structure. The major limitation of such POM catalysts is their low surface area, high solubility in polar solvents, and thermal instability. Therefore, several attempts have been made to transfer the homogeneous state into a heterogeneous states by supporting the POMs on different carriers.15,16 Kim and co-workers reported aH5PMo10V2O40 supported on silica ball/mesoporous carbon/SBA-15 as the heterogeneous catalyst in the CWAO of phenol,13 using H2O2 as an oxidant. The alternative is to integrate POMs into polymer matrices, which is a good method for creating novel POM-containing polymers, possessing both the unique properties of POM clusters and the favorable processability of polymers.17 Polymeric fiber membranes have also been shown to be very useful for heterogeneous catalysis18 and micro-filters,19 which suggests a potential application for removing organic pollutions from wastewater. More importantly, one-dimensional (1D) nanofibers can make catalytic fiber membranes available for a continuous mode by passing the pollutant solution through the membrane, making them suitable for industrial applications.
Electrospinning is an effective and easily controllable way to fabricate POM/polymer nanofibers compared with other methods.20 Electrospinning requires only a simple mixed solution of polymer and POM precursors to fabricate 1D nanofibers with long length, uniform diameter, diverse composition, and high surface area, which can have several applications such as catalyst supports. To date, there have been few reports on the construction of one-dimensional POM nanofibers using an electrospinning method,21–24 in which only one had been used for the photodegradation of organic dyes. The aim of the present work is to fabricate POM-functionalized fiber membranes using a simple procedure and to develop a novel technology for the decontamination of industrial wastewaters and polluted water resources based on POMs. In this study, the electrospinning technique was used to fabricate POM nanofibers using an insoluble polymer as a support. The hybrid catalysts were used to evaluate the catalytic activity for the CWAO of phenol in a continuous mode. To date, there has been no report on the degradation of phenol using a continuous mode based on POM catalysts.
2. Results and discussion
2.1. Characterization of K5PMo10V2O40/PAN nanofibers
From the results of elemental analysis, the contents of K5PMo10V2O40/PAN were determined to be Mo, 7.05; P, 0.22; V, 0.75; and C, 49.45%. The molar ratio of Mo![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P
P![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) V was 10
V was 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, showing that POMs were intact in the polymer fibers. The content of K5PMo10V2O40 in PAN was 14.2%.
2, showing that POMs were intact in the polymer fibers. The content of K5PMo10V2O40 in PAN was 14.2%.
The IR spectrum of the POM nanofibers was investigated (Fig. 1a). Peaks in the range of 600–1100 cm−1 could be easily distinguished at 1084, 949, 852, and 797 cm−1. Its parent [PMo10V2O40]5− gives four characteristic peaks, including P–O stretching (∼1064 cm−1), M–Oter stretching (∼964 cm−1), Mo–Oc–Mo stretching of inter bridges between corner-sharing MoO6 octahedra (∼876 cm−1), and Mo–Oe–Mo stretching of intra bridges between edge-sharing MoO6 octahedra (∼814 cm−1). From the IR results, the POMs maintained their original Keggin structure after being loaded onto the PAN. The peak at 2294 cm−1 was attributed to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N, corresponding to PAN. Some shifts indicated the interaction between POM and PAN polymer.
N, corresponding to PAN. Some shifts indicated the interaction between POM and PAN polymer.
The 31P MAS NMR spectrum of K5PMo10V2O40/PAN was recorded (Fig. 1b). Two signal peaks are observed at 5.61 and 6.56 ppm for K5PMo10V2O40/PAN, respectively. Compared to its parent K5PMo10V2O40 (δ = 4.75 ppm), the splitting of one peak into two was attributed to the interaction between K5PMo10V2O40 and the polymer PAN.
Fig. 1c is a typical SEM image of the K5PMo10V2O40/PAN nanofibers with an average diameter of 100 nm. From EDS (Fig. 1d), the molar ratio of P, Mo and V was determined to be 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2. From the high resolution TEM image (Fig. 1e), it can be seen that K5PMo10V2O40 was dispersed uniformly around the polymer.
2. From the high resolution TEM image (Fig. 1e), it can be seen that K5PMo10V2O40 was dispersed uniformly around the polymer.
From the result of elemental analysis (Mo, 8.39; P, 0.24; V, 0.45; and C, 47.23%), the molar ratio of Mo![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P
P![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) V was determined to be 11
V was determined to be 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, showing that the original structure was intact. The content of K4PMo11VO40 in PAN was 16.0%. From the SEM image of K4PMo11VO40/PAN (Fig. 1f), it can be seen that the diameter was about 100 nm.
1, showing that the original structure was intact. The content of K4PMo11VO40 in PAN was 16.0%. From the SEM image of K4PMo11VO40/PAN (Fig. 1f), it can be seen that the diameter was about 100 nm.
2.2. CWAO activity of K5PMo10V2O40/PAN nanofibers
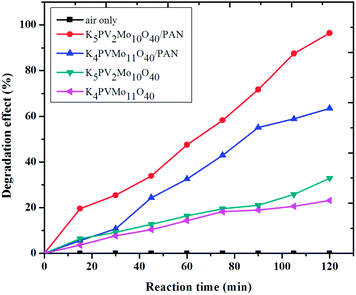
First, in order to determine the catalytic activity of K5PMo10V2O40/PAN, the degradation of phenol by different catalysts was carried out (Fig. 2) under flowing oxygen in a flash reactor. No phenol degradation was detected without any catalyst, which implied that the oxidation ability of O2 at room temperature was limited. The catalytic activity was in the following order: K4PMo11VO40 < K5PMo10V2O40 < K4PMo11VO40/PAN < K5PMo10V2O40/PAN. The potassium of POMs is insoluble in water at room temperature and has a small surface area of 4.3 m2 g−1, which acted as a heterogeneous catalyst. K5PMo10V2O40 exhibited low activity in the degradation of phenol by air. The highest activity was obtained by K5PMo10V2O40/PAN, which exhibited 96.4% degradation efficiency for 0.72 mM phenol, which is three times higher than that of K5PMo10V2O40. The higher activity of K5PMo10V2O40/PAN was attributed to the high dispersion of K5PMo10V2O40 on the PAN nanofibers, high surface area, and the enhanced adsorption of phenol by PAN. From its XRD (Fig. S1†), no POM diffraction peaks were observed, indicating a good dispersion of K5PMo10V2O40 around the PAN nanofibers. The image of high resolution TEM also supported the uniform dispersion of K5PMo10V2O40. The surface area of K5PMo10V2O40/PAN was about 361.8 m2 g−1 higher than that of K5PMo10V2O40. It is known that the nanofiber membrane can adsorb certain substrates. The adsorption capacity on the oxidation catalyst is a key factor for the degradation rate in the air oxidation process,25 which provides enhanced mass transport for oxygen molecules into and out of the pore structure.26 The adsorption of phenol by K5PMo10V2O40/PAN and K5PMo10V2O40 were about 24.5% and 3.6%, respectively. The increasing adsorption of phenol was attributed to the effect of the PAN (23.9%). Therefore, it can be concluded that the adsorption of phenol was enhanced by the assistance of the PAN nanofibers, enhancing the degradation rate. In addition, the vanadium content in POMs also influenced the degradation of phenol; monovanadium POM K4PMo11VO40/PAN exhibited lower activity than divanadium K5PMo10V2O40/PAN. Cyclic voltammetry of K4PMo11VO40/PAN and K5PMo10V2O40/PAN provided the first redox potentials for the VO2+/VO2+ couple in water as 0.46 and 0.47 V, respectively. Therefore, the different catalytic performance was assumed to correspond to the redox potential with respect to vanadium substitution, similar to our previous report.27It is important to clarify whether the removal of phenol by K5PMo10V2O40/PAN was by adsorption or oxidation. Therefore, the degradation products during the reaction were determined using ion chromatography and HPLC (Fig. S2†). It can be seen that phenol was first oxidized into hydroquinone, catechol, and then into p-benzoquinone, o-benzoquinone, and maleric acid. The final degradation products were CO2 and water, which could be determined by Ca(OH)2. These results determined that the removal of phenol was by an oxidative process in air and was catalyzed by K5PMo10V2O40/PAN.
To study the effect of initial concentration on the degradation of phenol, experiments were carried out with different concentrations of phenol from 0.72 mM to 3.60 mM (Fig. S3a†). It was observed that with an increase in phenol concentration, the degradation efficiency decreased, which showed that the removal of phenol was dependent on its initial concentration. The decrease in activity was attributed to the decreasing ratio of phenol to catalyst, showing that the limitation of catalytically active sites hindered further adsorption of phenol, resulting in a lower activity. In addition, the influence of temperature on degradation efficiency was studied (Fig. S3b†). It can be seen that the degradation efficiency increased with increasing temperature from 0 °C to 25 °C. Meanwhile, the catalytic activity of K5PMo10V2O40/PAN was high even at a lower temperature, where the degradation efficiency was 82.4% for a 120 min reaction. The amount of catalyst also influenced the degradation of phenol (Fig. S3c†); for example, 0.2 g of K5PMo10V2O40/PAN could produce 96.4% removal of phenol.
Based on the above results, it can be concluded that the removal of phenol by K5PMo10V2O40/PAN and oxygen proceeded in two steps. Initially, the reactants (phenol and dioxygen) reached the surface of nanofibers and were adsorbed on the surface by hydrogen bonding interactions between the phenol hydroxyl groups and the POM oxygen atoms. Second, the phenol molecules were oxidized by free radicals, which has been already reported by our group.27 This process was influenced by the adsorption of phenol on the nanofibers, the generation of free radicals, use of the catalyst or oxygen, and temperature.
2.3. The degradation of phenol using continuous mode
The degradation of phenol under continuous mode is more convenient for coupling and separation. Therefore, the degradation efficiency of phenol using a continuous mode at different flow rates is summarized in Fig. 3. The results indicated that on decreasing the flow rate from 14.4 to 4.8 mL min−1 the removal efficiency increased from 70.7% to 100% for nine cycles. The cycling times of the total degradation of phenol were different when using the different flow rates 14.4, 9.6, and 4.8 mL min−1 with 9, 12 and 14 cycles, respectively. Using a slow flow rate, the residence time of the reactant in the reactor increased, which resulted in high degradation efficiency. Compared to the result of placing all the catalyst in one tube (the final decolorization effect was about 87.8%), the tandem of nine glass tubes might provide better performance. This could be attributed to the residence time of phenol molecules on the surface of the catalyst. Under our experimental conditions, the amount of the catalyst used was small, thus all the catalyst was placed in one tube; the time of the phenol molecules flowing across the catalyst fibers was relatively shorter than that in nine tandem tubes. Therefore, the continuous mode might be more available for treating phenol in waste water. | ||
| Fig. 3 Effect of the flow rate of phenol solution on degradation under a continuous mode (room temperature, 100 mL of 0.72 mM phenol solution). | ||
The initial concentration of phenol also influenced the degradation effect; experiments were carried out with different concentrations of phenol from 0.72 mM to 3.6 mM (Fig. 4) at a flow rate of 4.8 mL min−1. It was found that with an increase in the concentration of phenol from 0.72 to 2.88 mM, the degradation efficiency decreased.
Mineralization of phenol in a continuous mode was studied by COD (chemical oxygen demand), and TOC (total organic carbon) assay. COD values were related to the total concentration of organics in the solution and the decrease of COD reflects the degree of mineralization.28 A significant decrease of COD was observed as 95.8%. The TOC reduction of phenol reached 94.5% after the ninth tube. Total mineralization could be achieved by K5PMo10V2O40/PAN and air, which showed that it was catalytically active and suitable for mineralization of phenol in a continuous mode.
The IR spectrum of K5PMo10V2O40/PAN after the reaction showed no changes, showing that it was stabile during the reaction. No peaks corresponded to phenol, indicating that no phenol was adsorbed on the surface of the catalyst. The phenol was totally degraded into CO2 and water. The leaching of K5PMo10V2O40 was 1.8 ppm in the continuous mode, showing little leaching of catalytic sites from the polymer support.
Reusability of the catalyst is very important for heterogeneous POM nanofibers. After the first catalytic run, the hybrid nanofibers were washed with distilled water in the reactor and dried under air for reuse. Two catalytic cycles were accomplished successfully with almost the same activity with no observable loss in performance.
3. Experimental
3.1. Materials
Polyacrylonitrile (PAN, Mw = 150![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) was purchased from Aldrich. Other reagents were of AR grade and were used without further purification. For oxidation degradation, 0.1 M stock solution of phenol was prepared and an aqueous solution of the required concentration was prepared from the stock solution. Molybdovanadophosphoric salts (K5PMo10V2O40 and K4PMo11VO40) were prepared according to the studies in ref. 29, and were characterized by IR spectroscopy.
000) was purchased from Aldrich. Other reagents were of AR grade and were used without further purification. For oxidation degradation, 0.1 M stock solution of phenol was prepared and an aqueous solution of the required concentration was prepared from the stock solution. Molybdovanadophosphoric salts (K5PMo10V2O40 and K4PMo11VO40) were prepared according to the studies in ref. 29, and were characterized by IR spectroscopy.
3.2. Apparatus
Elemental analysis was carried out using a Leeman Plasma Spec (I) ICP-ES (Leeman LABs. Inc.). IR spectra (4000–500 cm−1) were recorded using KBr discs on a Nicolet Magna 560 IR spectrometer. SEM was operated using XL30 ESEM FEG at 25 kV (Philips XL-30). The high resolution TEM image was measured on a JOEL JEM-2100F microscope. XRD patterns of the sample were collected on a Rigaku Dmax 2000 X-ray diffractometer with Cu Kα radiation (λ = 0.154178 nm) (Rigaku Corporation). The 31P MAS NMR measurements were obtained using a Bruker AM500 spectrometer at 202.5 MHz. Nitrogen porosimetry was performed on a Micromeritics ASAP 2010 instrument. Surface areas were calculated using the BET equation. The analysis of phenol and its intermediates during the reaction was performed by high performance liquid chromatography (HPLC, Shimadzu LC-20A) with a UV detector using a Shim-pack VP-ODS (4.6 mm × 250 mm, 5 μm) column. A DX-300 ion chromatography (IC) equipped with a conductivity detector and an ICE-ASI anion column was also used to determine the changes in the concentrations of the intermediates and final products. Total organic carbon (TOC) was monitored using a Shimadzu TOC-VCPH total organic carbon analysis system. Chemical oxygen demand (COD) was determined using a closed reflux colorimetric method by a 756 CRT UV-vis spectrophotometer operating at a wavelength of 600 nm. The leaching concentrations of K5PMo10V2O40 during the reaction were also measured by analyzing the dissolved concentration of Mo in aqueous solution using a Leeman Plasma Spec (I) ICP-ES. The cyclic voltammograms were recorded with an RDK-RW-117 X-Y recorder (Japan).3.3. Preparation of K5PMo10V2O40/PAN nanofibers
Nanofibers of K5PMo10V2O40/PAN were prepared by an electrospinning method. A 10 wt% polymer solution was prepared by adding 1.85 g PAN into 16.65 g of (N,N-dimethylformamide) DMF with stirring for 5 h at 60 °C. 0.27 g of K5PMo10V2O40 was added into the abovementioned solution with gentle stirring for 12 h to obtain a homogeneous hybrid sol for further electrospinning.K4PMo11VO40/PAN was prepared using the same method except for using K4PMo11VO40 instead of K5PMo10V2O40.
3.4. Catalytic activity
For slurry-type reactors, a measured amount of catalyst was suspended in a fresh aqueous phenol solution (C0 = 0.72 mM, 100 mL, pH = 1.7) in a three-necked glass flask at ambient temperature (25 °C). The air was injected into the bottom of the suspension at a flow rate of 0.04 m3 h−1. This process directly used oxygen dissolved in the aqueous solution as an oxidant. Oxygen concentration depends on the oxygen solubility at room temperature and atmospheric pressure. Samples were withdrawn periodically for measurement. After completion, the catalyst was separated by filtration and washed with water three times for reuse.The continuous experiments were carried out in a continuous-flow reactor, which is shown in Scheme 1. The reactor comprised five glass tubes (inner diameter 25 mm, outer diameter 26.6 mm), which were serially connected by transparent polyethylene tubes from top to bottom. Each glass tube was equipped with a permeable plug at the base of a glass tube. The K5PMo10V2O40/PAN (0.2 g) fibers were fixed on top of the plug. The oxygen-saturated solution was obtained by bubbling with air for at least 20 min. 100 mL of phenol solution (0.72 mM) was then forced through the membrane at different rates at room temperature (25 °C). A sample was collected from the outlet of each glass column.
3.5. Adsorption experiments
For the adsorption measurements, 100 mL, 0.72 mM of freshly prepared phenol solution was passed through K5PMo10V2O40/PAN fibers (0.2 g) at a flow rate of 4.8 mL min−1 by bubbling with N2 to drive out the dissolved O2. When all the solution had passed through the membrane, a sample was taken to determine phenol concentration.4. Conclusions
The present study reports the fabrication of POMs/PAN nanofibers by an electrospinning technique; these nanofibers were employed as catalysts for the degradation of phenol in a continuous mode at room temperature. K5PMo10V2O40/PAN nanofibers exhibited excellent catalytic activity due to its unique properties such as high dispersion of POM, high surface area, and enhanced adsorption of phenol. Phenol molecules were completely mineralized into small inorganic species, CO2 and water using nine tandem glass tubes. This catalytic process demonstrated a commercial and green chemical pathway with potential industrial application for phenol degradation.Acknowledgements
This work was supported by the National Natural Science Foundation of China (no. 51078066), the major projects of Jilin Provincial Science and Technology Department (20086035, 20100416, and 201105001).References
- N. S. Inchaurrondo, P. Massa, R. Fenoglio, J. Font and P. Haure, Chem. Eng. J., 2012, 198–199, 426–434 CrossRef CAS PubMed.
- M. A. Baboli, Environ. Toxicol. Pharmacol., 2012, 34, 826–831 CrossRef PubMed.
- R. Mu, Z. Xu, L. Li, Y. Shao, H. Wan and S. Zheng, J. Hazard. Mater., 2010, 176, 495–502 CrossRef CAS PubMed.
- E. V. Rhokina, E. Repo and J. Virkutyte, Ultrason. Sonochem., 2010, 17, 541–546 CrossRef PubMed.
- M. H. El-Naas, S. Al-Zuhair and S. Makhlouf, Chem. Eng. J., 2010, 160, 565–570 CrossRef CAS PubMed.
- F. Arena, C. Italiano, A. Raneri and C. Saja, Appl. Catal., B, 2010, 99, 321–328 CrossRef CAS PubMed.
- L. T. Lai, C. C. Lee, G. L. Huang, Y. Y. Shu and C. B. Wang, Appl. Catal., B, 2008, 78, 151–157 CrossRef PubMed.
- B. Y. Jibril, A. Y. Atta, Y. M. Al-Waheibi and T. K. Al-Waheibi, J. Ind. Eng. Chem., 2013, 19, 1800–1804 CrossRef CAS PubMed.
- M. Abecassis-Wolfovich, M. V. Landau, A. Brenner and M. Herskowitz, Ind. Eng. Chem. Res., 2004, 43, 5089–5097 CrossRef CAS.
- M. Abecassis-Wolfovich, R. Jothiramalingam, M. V. Landau, M. Herrskowitz, B. Viswanathan and T. K. Varadarajan, Appl. Catal., B, 2005, 59, 91–98 CrossRef CAS PubMed.
- F. Arena, J. Negro, G. Trunfio and A. Parmaliana, Ind. Eng. Chem. Res., 2007, 46, 6724–6731 CrossRef CAS.
- F. Arena, G. Trunfio, J. Negro and L. Spadaro, Appl. Catal., B, 2008, 85, 40–47 CrossRef CAS PubMed.
- S. V. Mayani, V. J. Mayani and S. W. Kim, Mater. Lett., 2013, 111, 112–115 CrossRef CAS PubMed.
- S. Zhao, X. H. Wang and M. X. Huo, Appl. Catal., B, 2010, 97, 127–134 CrossRef CAS PubMed.
- K. M. Parida and S. Mallick, J. Mol. Catal. A: Chem., 2008, 279, 104–111 CrossRef CAS PubMed.
- K. Y. Lee, S. Oishi, H. Igarashi and M. Misono, Catal. Today, 1997, 33, 183–189 CrossRef CAS.
- W. K. Miao, Y. K. Yan, X. L. Wang, Y. Xiao, L. J. Ren, P. Zheng, C. H. Wang, L. X. Ren and W. Wang, ACS Macro Lett., 2014, 3, 211–215 CrossRef CAS.
- H. Yu, J. Zhu, Y. G. Li, Q. H. Zhang and M. F. Zhu, Mater. Lett., 2012, 74, 247–249 CrossRef CAS PubMed.
- K. H. Choo, D. I. Chang, K. W. Park and M. H. Kim, J. Hazard. Mater., 2008, 152, 183–190 CrossRef CAS PubMed.
- A. Formhals, US. Pat. Specif., 1975504, 1934, vol. 1, pp. 504–975.
- C. H. Sui, C. Li, X. H. Guo, T. X. Cheng, Y. K. Gao, G. D. Zhou, J. Gong and J. S. Du, Appl. Surf. Sci., 2012, 258, 7105–7111 CrossRef CAS PubMed.
- B. Ding, C. Li, S. Fujita and S. Shiratori, Colloids Surf., A, 2006, 284–285, 257–262 CrossRef PubMed.
- J. Gong, X. Li, B. Ding, D. R. Lee and H. Y. Kim, J. Appl. Polym. Sci., 2003, 89, 1573–1578 CrossRef CAS.
- J. Gong, C. Shao, Y. Pan, F. M. Gao and L. Y. Qu, Mater. Chem. Phys., 2004, 86, 156–160 CrossRef CAS PubMed.
- M. W. Xue, X. D. Gu, J. P. Chen, H. L. Zhang and J. Y. Shen, Thermochim. Acta, 2005, 434, 50–54 CrossRef CAS PubMed.
- Y. Yang, Q. Y. Wu, Y. H. Guo, C. W. Hu and E. B. Wang, J. Mol. Catal. A: Chem., 2005, 225, 203–212 CrossRef CAS PubMed.
- S. Zhao, X. H. Wang and M. X. Huo, Appl. Catal., B, 2010, 97, 127–134 CrossRef CAS PubMed.
- M. A. Behnajady, N. Modirshahla, N. Daneshvar and M. Rabbani, J. Hazard. Mater., 2007, 140, 257–263 CrossRef CAS PubMed.
- G. A. Tsigdinos and C. J. Hallada, Inorg. Chem., 1968, 7, 437–441 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra01434k |
| This journal is © The Royal Society of Chemistry 2014 |