Spectroscopic and electrochemical studies on the evaluation of the radical scavenging activities of luteolin by chelating iron†
Ai-Hong Yang*a,
Xue-Ying Shib,
Xue Lia,
Fang-Fang Lib,
Qin-Qin Zhangb,
Shu-Xin Jiangb,
Jian-Zhong Cuib and
Hong-ling Gaob
aTianjin Key Laboratory of Chemistry and Analysis of Chinese Materia Medica, College of Chinese Materia Medica, Tianjin University of Traditional Chinese Medicine, Tianjin 300193, P. R. China. E-mail: yah408@163.com
bDepartment of Chemistry, School of Science, Tianjin University, and Collaborative Innovation Center of Chemical Science and Engineering (Tianjin), Tianjin 300072, China
First published on 29th May 2014
Abstract
Luteolin, 5,7,3′,4′-tetrahydroxylflavone, which is one of the most common dietary flavonoids, exists in many types of plants including fruits, vegetables and medicinal herbs. Spectroscopic studies (UV-visible, ESI-MS) and cyclic voltammetry (CV) were employed to study the relevant interaction of luteolin and Fe(III) in ethanol, as well as to evaluate the antioxidant activities of luteolin and its complexes against free radical-mediated damage, such as hydroxyl radicals (˙OH) and 1,1-diphenyl-2-picrylhydrazyl (DPPH) radicals. Luteolin can form a luteolin–Fe(III) complex with the ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and coordinate to the Fe(III) ion at the 3′,4′-dihydroxyl group in ring B in the luteolin molecule. Luteolin strongly inhibits Fenton reaction via a combined effect of Fe(II) chelation and radical scavenging activities. The antioxidant activities of luteolin and its Fe(III) complex were evaluated by using the DPPH radical scavenging method. Luteolin shows better antioxidant activity than the luteolin–Fe(III) complex, which is also verified from the electrochemical point of view. The experimental results can be rationalized in terms of structural features governing antioxidant behavior i.e. substitution pattern of ring B in the luteolin molecule.
1 and coordinate to the Fe(III) ion at the 3′,4′-dihydroxyl group in ring B in the luteolin molecule. Luteolin strongly inhibits Fenton reaction via a combined effect of Fe(II) chelation and radical scavenging activities. The antioxidant activities of luteolin and its Fe(III) complex were evaluated by using the DPPH radical scavenging method. Luteolin shows better antioxidant activity than the luteolin–Fe(III) complex, which is also verified from the electrochemical point of view. The experimental results can be rationalized in terms of structural features governing antioxidant behavior i.e. substitution pattern of ring B in the luteolin molecule.
1. Introduction
Free radicals and reactive oxygen species (ROS) are associated with many pathological conditions such as aging, ischemia-reperfusion, cardiovascular diseases, immunodeficiency diseases, heart diseases and cancer.1–3 The superoxide anion (O2˙−), the hydroxyl radical (˙OH) and hydrogen peroxide (H2O2) are the most common ROS. As the most active member of ROS, ˙OH can induce oxidative stress and then cause damage to cell membranes and DNA strand breaks, as well as membrane lipid peroxidation with subsequent decreases in membrane fluidity.4,5 Therefore, ˙OH-scavenging is significant in biochemistry.6In order to offset the damages of ROS, organisms have developed a variety of defence mechanisms including antioxidase (superoxide dismutase, catalase and glutathione peroxidase), active proteins (ferritin and transferrin) and water- or lipid-soluble antioxidants.7 Furthermore, some external factors, such as flavonoids, as chemical regulator of certain enzymes and transporters, is indispensable.8 In recent decades, flavonoids have attracted much more attention since they exert multiple biological effects such as anti-inflammatory, antimicrobial, anticancer and cardiovascular protection, which mainly arise from their antioxidant effect.9–11 Previous studies in vitro have demonstrated that the antioxidant activities of the flavonoids act as hydrogen-donating or electron-donating free radical scavengers.12,13 The antioxidant effect of flavonoids is supposedly due to their free radical scavenging activities.14
Generally, the ability of flavonoids to chelate metals is assumed to be very important for their antioxidant activity. Some flavonoids, such as quercetin,15–17 fisetin,18 baicalein,19 morin20 and naringin21 can chelate potentially toxic iron ions, which have been supported by many researchers. Furthermore, some flavonoids, quercetin,15 baicalein19 and morin20 strongly prevent the generation of free radical from Fe-promoted Fenton reaction.
Iron is an essential element for most organisms on earth including most bacterial species, animals and especially human beings due to its important role in oxygen transport, a variety of cellular processes like respiration and DNA synthesis and catalytic activity of many enzymes.22–24 However, it is toxic when it is overload, which can increase oxidative stress and reactive oxygen species, in turn causing the lipid peroxidation, damages to proteins and nucleic acids (mainly causing mutations in DNA or cell death).25 Especially the overload iron can catalyze the Fenton reaction, which has a direct relationship with inflammation. Inflammation is one of the body's valuable defense mechanisms that can combat foreign substances, guard against infection and help healing injury. There are several hypotheses that explain the process of acute inflammation including over-production of ROS and reactive nitrogen species (RNS), complex array of enzymes activation, and release of several inflammatory molecules and pro-inflammatory cytokines.26,27 In which, ˙OH, as the most active member of ROS, can induce oxidative stress.
Luteolin is a common flavonoid with biological effects such as antioxidant, anti-inflammation, antiallergic, anticancer and neuroprotective activities.28–30 It possesses two metal ions chelating sites: the 3′,4′-dihydroxy group in ring B and the 5-hydroxy and 4-carbonyl group in ring C (Scheme 1), which is predicted to chelate iron and then scavenge free radicals.
However, up to now, there are few studies on antioxidant effect about Fe(III) and luteolin, except for some spectroscopic31–33 and anti-inflammatory34 studies about other metal complexes of luteolin.
This paper addressed in vitro experimental research regarding the iron chelation of luteolin in ethanol and their antioxidant activities. The UV-vis and ESI-MS spectroscopy were applied to investigate the stoichiometry and chelation sites of luteolin–Fe(III) complex. The effect of luteolin in attenuating Fenton reaction was studied under physiologically relevant conditions. The antioxidant activities of luteolin and its Fe(III) complex were evaluated by DPPH decay method and from an electrochemical point of view as well.
2. Reagents and methods
2.1 Reagents
Luteolin, Fe(NO3)3·9H2O and ethanol were used for synthesis of luteolin–Fe(III) complex. Ethylenediaminetetraacetic acid (EDTA), FeSO4·7H2O, hydrogen peroxide, 2-deoxyribose, thiobarbituric acid (TBA) and 1,1-diphenyl-2-picrylhydrazyl (DPPH) were used for antioxidant tests. All reagents were purchased from commercial sources. The solutions used for measurements were freshly prepared before experiments and were used immediately.2.2 Measurements
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and 1
2 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) were prepared in the similar method.
3) were prepared in the similar method.The pH value of the ethanol solution of luteolin is 5.6, and each complex is formed under this condition without adjusting the pH value. Luteolin acts as a weak polybasic acid and therefore pH value plays an important role in its coordination with metal ions. The chelating ability of luteolin decreases as the pH decreases since the inhibition on the deprotonation. The coordination ability enhances with the increase of pH, however, it is prone to precipitation with a higher pH value of the solution. So the complexes have been obtained without adjusting the pH value.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 of mixture of iso-propyl alcohol and 0.1% acetic acid aqueous solution, and the flow rate was 400 μL min−1. The mass spectrometer was run in positive ESI mode with the mass/charge (m/z) in the range 100–3200 m/z.
50 of mixture of iso-propyl alcohol and 0.1% acetic acid aqueous solution, and the flow rate was 400 μL min−1. The mass spectrometer was run in positive ESI mode with the mass/charge (m/z) in the range 100–3200 m/z.The reaction was monitored in the presence or absence of Fe(II) chelators (luteolin and EDTA). The Fe(II) solution (concentration ranges 0.05–0.5 mmol L−1 prepared in 0.1 mol L−1 HCl, 1 mL), 2-deoxyribose (40 mmol L−1, 0.3 mL) and H2O2 (4 mmol L−1, 0.15 mL) were added to the buffered solutions (pH = 7.2, 1 mL). The reactions were allowed to run for 10 min at room temperature and then stopped by adding 1 mL 4% phosphoric acid (v/v) followed by 1 mL 0.5% TBA (w/v, in 50 mmol L−1 NaOH). After boiling the mixture for 15 min, the visible absorbance of the formed TBA–MDA was measured at 532 nm. The second step of the reaction was carried out using the same procedure but with the addition of Fe(II) chelators (luteolin and EDTA) (0.4 mmol L−1, 1 mL) after the addition of hydrogen peroxide. Each measurement was repeated three times. Then we plotted the curve using the average of three groups. Three sets of data are listed in Table S6 in ESI.†
Ethanol solutions containing different concentrations of luteolin (0.05–0.3 mmol L−1, 1 mL) were added to DPPH ethanol solution (0.2 mmol L−1, 1 mL), then the total volume of each solution was added to 5 mL by ethanol. The same procedure was carried out using luteolin–Fe(III) complex instead of luteolin. Each measurement was repeated three times. Then we plotted the curve using the average of three groups. Three sets of data are listed in Tables S7–S9 in ESI.†
Free radical scavenging ability is defined as the amount of antioxidant necessary to decrease the initial DPPH concentration by 50% (EC50). For each antioxidant, different concentrations were tested (expressed as molar ratio of antioxidant and DPPH).
3. Results and discussion
3.1 UV-visible study
The UV-vis spectra of luteolin and different luteolin–Fe(III) systems (Lu–Fe = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 1
2, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) are shown in Fig. 1. Luteolin has two major absorption bands in UV-vis region, at 351 nm (band I) assigned to the π → π* transitions in B ring system (cinnamoyl system) and at 255 nm (band II) representing the A ring system (benzoyl system). After addition of Fe(III) to the ethanol solution of luteolin, it produces large bathochromic-shift in band I, Δλmax = 40 nm, giving simultaneous rise to a new absorption band that can be attributed to the complex formation. The isosbestic point which is present in 372 nm indicates equilibrium between free and complexed luteolin. Band II of luteolin spectrum bathochromically shifts to 269 nm in the presence of Fe(III). The molar ratio plot at 391 nm indicates the formation of 1
3) are shown in Fig. 1. Luteolin has two major absorption bands in UV-vis region, at 351 nm (band I) assigned to the π → π* transitions in B ring system (cinnamoyl system) and at 255 nm (band II) representing the A ring system (benzoyl system). After addition of Fe(III) to the ethanol solution of luteolin, it produces large bathochromic-shift in band I, Δλmax = 40 nm, giving simultaneous rise to a new absorption band that can be attributed to the complex formation. The isosbestic point which is present in 372 nm indicates equilibrium between free and complexed luteolin. Band II of luteolin spectrum bathochromically shifts to 269 nm in the presence of Fe(III). The molar ratio plot at 391 nm indicates the formation of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 luteolin–Fe(III) complex (Fig. 2). The measurement was repeated three times. Then we plotted the curve using the average of three groups. Three sets of data are listed in Table S10 in ESI.† The stability constant value of luteolin–Fe(III) complex (log
1 luteolin–Fe(III) complex (Fig. 2). The measurement was repeated three times. Then we plotted the curve using the average of three groups. Three sets of data are listed in Table S10 in ESI.† The stability constant value of luteolin–Fe(III) complex (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) β = 8.4), calculated by molar ratio method, is a little larger than the constant value of morin–Fe(III),24 while smaller than those of quercetin–Fe(III)15 and fisetin–Fe(III),18 indicating luteolin–Fe(III) complex less stable, which can also be seen in Fig. S1 in ESI,† luteolin can recover from luteolin–Fe(III) complex after the addition of EDTA.
β = 8.4), calculated by molar ratio method, is a little larger than the constant value of morin–Fe(III),24 while smaller than those of quercetin–Fe(III)15 and fisetin–Fe(III),18 indicating luteolin–Fe(III) complex less stable, which can also be seen in Fig. S1 in ESI,† luteolin can recover from luteolin–Fe(III) complex after the addition of EDTA.
3.2 IR analysis
The curves of IR spectra of luteolin and its Fe(III) complex have been shown in Fig. S2 and S3 in ESI.† By comparing the absorption data of the complex and ligand, the following information can be obtained: the wide absorption peaks around 3420 cm−1 in the IR spectra of luteolin and complex assign to the vibration of phenolic hydroxyl groups. The absorption peak at 1659 cm−1 in luteolin suggests the stretching vibration of carbonyl group, which shifts to 1624 cm−1 in the complex. The benzene stretching vibration absorption peak at 1608 cm−1 in luteolin shifts to 1570 cm−1, which is due to enhanced conjugation effect of the benzene ring after coordination. The absorption peak at 1266 cm−1 in luteolin shifts to 1253 cm−1, which indicates phenolic hydroxyl groups involved in coordination. The absorption peak at 1164 in luteolin and complex assigns to the C–O–C stretching vibration. The peak appears at 639 cm−1 indicating the O–Fe(III) bond.3.3 ESI-MS
Due to its high sensitivity, high specificity and great accuracy in the mass spectrum assignments, the ESI-MS technique has been widely used to characterize metal complexes, which can allow the user to obtain the stoichiometry of different species existing in dilute solutions. The soft ionization–desorption process actually allows the integrated species transfer from the solution into the gas phase where the mass assignment, together with MS2 experiments and simulation processes, give direct information on the metal complex stoichiometry.38,39 The ESI-MS experiments are performed on the luteolin–Fe(III) solution in the positive ion mode to verify the complex formation (Fig. 3). The most intense signals fall at m/z of 402.7, 431.6 and 448.8. The peak detected at m/z = 402.7 is assigned to the ion [(Lu − 2H)2− + Fe3+ + NO3− + H+]. The peak detected at m/z = 431.6 is attributed to the ion [(Lu − 2H)2− + Fe3+ + 2CH3CH2OH]. The peak detected at m/z = 448.8 is attributed to the ion [(Lu − 2H)2− + Fe3+ + NO3− + CH3CH2OH + H+]. When the ratios of Lu–Fe(III) are 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and 1
2 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, all the above three peaks can be detected with different intensity (Fig. S4 and S5 in ESI†). The most intense signals correspond to the formation of a 1
3, all the above three peaks can be detected with different intensity (Fig. S4 and S5 in ESI†). The most intense signals correspond to the formation of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 luteolin–Fe(III) complex. Peaks detected at m/z = 267.94, 388.29, 582.76 and 704.26 correspond to the signals of the solvent components.
1 luteolin–Fe(III) complex. Peaks detected at m/z = 267.94, 388.29, 582.76 and 704.26 correspond to the signals of the solvent components.
3.4 2-Deoxyribose degradation test
The oxidative degradation of 2-deoxyribose induced by Fenton reagents is presented in Fig. 4. In order to study the mechanism of luteolin scavenging ˙OH generated in Fenton reaction, the experiments are tested in the absence or presence of the known iron chelator EDTA. As shown curves (b) and (c) in Fig. 4, EDTA (0.4 mmol L−1) and luteolin (0.4 mmol L−1) inhibit 2-deoxyribose degradation with the inhibition rate of 88.1% and 60.1%, respectively. The higher inhibition rate of EDTA shows that: (1) EDTA is a stronger iron-chelator than luteolin; (2) EDTA–Fe(II) complexation is the key-factor in attenuating the Fenton reaction. As shown in curves (c) and (d) in Fig. 4, the joint addition of EDTA (0.4 mmol L−1) and luteolin (0.4 mmol L−1) can inhibit 2-deoxyribose degradation with an efficiency of 95.1%, higher than that of EDTA. The higher inhibition rate of EDTA and luteolin shows that luteolin acts as a radical scavenger rather than an iron chelator in the presence of the stronger iron chelator EDTA. It is concluded that luteolin strongly attenuates the Fenton reaction via a combined effect of iron-chelation and radical scavenging ability. Luteolin can strongly inhibit Fenton reaction, indicating its anti-inflammatory activity. Luteolin can scavenge ROS and suppress the LPS-activated nitric oxide production in activated macrophages.40,41 Antioxidant and inhibitory effects on inflammation-inducing enzymes (cyclooxygenase, lipoxygenase) of luteolin and the subsequent inhibition of inflammatory mediator (leukotrienes, prostaglandins) can contribute to its anti-inflammatory activity.42,43 | ||
| Fig. 4 Absorbance of malonaldehyde–TBA complex at 532 nm at various Fe(II) concentrations in the presence or absence of EDTA and luteolin. | ||
3.5 DPPH test
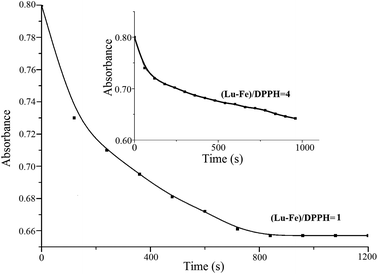
Fig. 5 shows DPPH visible absorbance decay after the addition of different concentrations of luteolin and its Fe(III) complex. The transfer of the labile H atoms in luteolin lasts longer about 480 s (Lu/DPPH = 0.15). When the ratio of Lu/DPPH rises, the time period becomes shorter, down to 420 s, 360 s, 300 s, 240 s corresponding to the ratios of Lu/DPPH 0.25, 0.75, 1, 1.5, respectively. Then all the curves reach a plateau, indicating that oxidation–degradation products of luteolin no more have H-transfer ability. As shown in Fig. 6, the transfer of the labile H atoms in luteolin–Fe(III) complex lasts longer than luteolin, up to 14 min at mole ratio (Lu–Fe)/DPPH = 1. At the (Lu–Fe)/DPPH = 4, the plateau cannot be reached even after longer kinetic runs (inset in Fig. 6), indicating prolonged H-transfer. So it is concluded that H-transfer becomes slower when the luteolin–Fe(III) complex formed.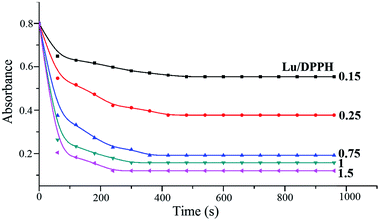 | ||
| Fig. 5 Decay of the visible absorbance (518 nm) of DPPH induced by different concentrations of luteolin. | ||
Moreover, the transfer of the labile H atoms becomes difficult in complex and the DPPH-scavenging ability becomes weaker. The maximum reduction of DPPH of luteolin is 84.9%, while that of luteolin–Fe(III) complex is only 18.5%. The EC50 value of luteolin is 0.249 (Fig. 7), showing that luteolin is a potent antioxidant. The EC50 value of luteolin–Fe(III) complex cannot be determined since the 50% reduction of DPPH cannot be reached under the investigated experiment conditions.
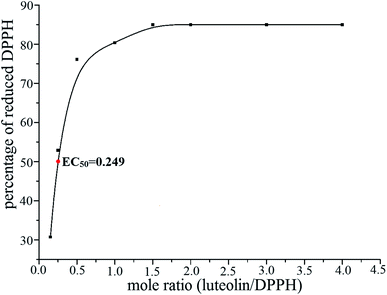 | ||
| Fig. 7 Percentage of the DPPH (0.2 mmol L−1) reduction as a function of different luteolin/DPPH mole ratios. | ||
The antioxidant capacity of flavonoids depends largely on their molecular structures. The 3′-OH and 4′-OH in ring B due to its better hydrogen donating properties may largely contribute to the high antioxidant ability of luteolin. Besides, the stable delocalization system, enabled by the C2–C3 double bond conjugated with the 4-keto group is a contributor to the high antioxidant activity of luteolin. Luteolin–Fe(III) is a weaker antioxidant than luteolin, which maybe the result of the catechol in ring B chelation with Fe(III). Therefore, it can be concluded that 3′-OH and 4′-OH are important in complexation and antioxidant activity of flavonoids, or that each –OH group strongly facilitates H-abstraction from the other by a combination of electronic and H-bonding effects.
3.6 Cyclic voltammograms
Electrochemical methods as an important class of diagnostic techniques have been widely used to establish interrelations between structure, oxidation potential and biological activity of electroactive species. These techniques highlight some advantages when compared with conventional chromatographic or spectroscopic methods, such as their operational stability, higher sensitivity, inherent specificity, short time of analysis and lower cost.44,45 Electrochemical methods have been recently introduced to characterize the electrochemical behavior of flavonoids.46 The electrochemical behavior of flavonoids can provide information about the free radical scavenging activity of natural polyphenols.Electrochemistry is the conceptual base of the selected antioxidant capacity assays. The results obtained by the DPPH radical-scavenging method are consistent with the reduction potentials. The reversible oxidation peak of luteolin occurs at 0.20 V which is corresponded to the oxidation of the 3′,4′-dihydroxyl group in ring B (Fig. 8). The corresponding reduction peak occurs at 0.23 V assigned to the formation of 3′,4′-diquinone. The reduction peak potential for luteolin–Fe(III) complex is observed at higher value (0.54 V) in relation to those of the free ligand (0.23 V).47 The higher reduction potential of luteolin–Fe(III) complex suggests that the coordination with Fe(III) makes the reduction process more difficult.
4. Conclusions
Luteolin can form luteolin–Fe(III) complex with the ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in ethanol. It has been analysed that the chelating sites are at the 3′,4′-dihydroxyl group in ring B. Luteolin strongly inhibits Fenton reaction may be due to a combined effect of chelation and radical scavenging activities. Luteolin is more effective than luteolin–Fe(III) complex in DPPH scavenging activity, which is verified from the point of cyclic voltammetry. There is a relationship between the antioxidant activity and the cyclic voltammetric reduction potential for flavonoids: the lower the reduction potential indicating the antioxidant much easier to emit electron and stronger reduction ability. The Fe-chelating ability of luteolin is assessed through the stability constant value, indicating the potentially effective iron chelator. Flavonoids with the “iron-binding motif” can be strong iron-chelator, which can help to modulate the bioactivity, bioavailability and concentration of iron and attenuate Fenton reaction.
1 in ethanol. It has been analysed that the chelating sites are at the 3′,4′-dihydroxyl group in ring B. Luteolin strongly inhibits Fenton reaction may be due to a combined effect of chelation and radical scavenging activities. Luteolin is more effective than luteolin–Fe(III) complex in DPPH scavenging activity, which is verified from the point of cyclic voltammetry. There is a relationship between the antioxidant activity and the cyclic voltammetric reduction potential for flavonoids: the lower the reduction potential indicating the antioxidant much easier to emit electron and stronger reduction ability. The Fe-chelating ability of luteolin is assessed through the stability constant value, indicating the potentially effective iron chelator. Flavonoids with the “iron-binding motif” can be strong iron-chelator, which can help to modulate the bioactivity, bioavailability and concentration of iron and attenuate Fenton reaction.
Acknowledgements
This work was supported financially by the National Natural Science Foundation of China (no. 21271137) and Specialized Research Fund for the Doctoral Program of Higher Education (no. 20121210120016).References
- M. Y. B. Çimen, Clin. Chim. Acta, 2008, 390, 1 CrossRef PubMed.
- F. Franzoni, R. Colognato, F. Galetta, I. Laurenza, M. Barsotti, R. D. Stefano, R. Bocchetti, F. Regoli, A. Carpi, A. Balbarini, L. Migliore and G. Santoro, Biomed. Pharmacother., 2006, 60, 453 CrossRef CAS PubMed.
- Y. Nakamura, S. Watanabe, N. Miyake, H. Kohno and T. Osawa, J. Agric. Food Chem., 2003, 51, 3309 CrossRef CAS PubMed.
- N. Yanai, S. Shiotani, S. Hagiwara, H. Nabetani and M. Nakajima, Biosci., Biotechnol., Biochem., 2008, 72, 3100 CrossRef CAS.
- G. X. Li, Z. Q. Liu and D. Wu, J. Phys. Org. Chem., 2009, 22, 883 CrossRef CAS.
- B. Halliwell, J. M. C. Gutteridge and O. I. Aruoma, Anal. Biochem., 1987, 165, 215 CrossRef CAS.
- B. Halliwell, R. Aeshbach, J. Loliger and O. I. Aruoma, Food Chem. Toxicol., 1995, 33, 601 CrossRef CAS.
- W. Jiang and M. Hu, RSC Adv., 2012, 2, 7948 RSC.
- T. F. Slater and M. N. Eakins, In New Trends in the Therapy of Liver Diseases, Karger, Basel, 1975, p. 84 Search PubMed.
- W. Bors and M. Saran, Free Radical Res. Commun., 1987, 2, 289 CrossRef CAS.
- A. Nègre-Salvayre and R. Salvayre, Free Radicals Biol. Med., 1992, 12, 101 CrossRef.
- C. A. Rice-Evans, N. J. Miller and G. Paganga, Free Radicals Biol. Med., 1996, 20, 933 CrossRef CAS.
- S. V. Jovanovic, S. Steenken, M. Tosic, B. Marjanovic and M. G. Simic, J. Am. Chem. Soc., 1994, 116, 4846 CrossRef CAS.
- V. A. Kostyuk, A. I. Potapovich, E. N. Strigunova, T. V. Kostyuk and I. B. Afanas'ev, Arch. Biochem. Biophys., 2004, 428, 204 CrossRef CAS PubMed.
- M. L. Guo, C. Perez, Y. B. Wei, E. Rapoza, G. Su, F. Bou-Abdallah and N. D. Chasteen, Dalton Trans., 2007, 4951 RSC.
- J. M. Dimitrić Marković, Z. S. Marković, T. P. Brdarić, V. M. Pavelkić and M. B. Jadranin, Food Chem., 2011, 129, 1567 CrossRef PubMed.
- P. Ryan and M. J. Hynes, J. Inorg. Biochem., 2008, 102, 127 CrossRef CAS PubMed.
- J. M. Dimitrić Marković, Z. S. Marković, T. P. Brdarić and N. D. Filipović, Dalton Trans., 2011, 40, 4560 RSC.
- C. A. Perez, Y. B. Wei and M. Guo, J. Inorg. Biochem., 2009, 103, 326 CrossRef CAS PubMed.
- J. M. Dimitrić Marković, Z. S. Marković, I. A. Pašti, T. P. Brdarić, A. Popović-Bijelić and M. Mojović, Dalton Trans., 2012, 41, 7295 RSC.
- G. C. Jagetia, T. K. Reddy, V. A. Venkatesha and R. Kedlaya, Clin. Chim. Acta, 2004, 347, 189 CrossRef PubMed.
- R. S. Eisenstein and K. P. Blemings, J. Nutr., 1998, 128, 2295 CAS.
- C. Ratledge and L. G. Dover, Annu. Rev. Microbiol., 2000, 54, 881 CrossRef CAS PubMed.
- P. J. Stephens, D. R. Jollie and A. Warshel, Chem. Rev., 1996, 96, 2491 CrossRef CAS PubMed.
- I. Fridovich, Science, 1978, 20, 875 Search PubMed.
- J. R. Vane and R. M. Bolting, Inflammation Res., 1995, 11, 1 CrossRef.
- K. Madhusudana, B. Shireesha, V. G. Naidu, S. Ramakrishna, B. Narsaiah, A. R. Rao and P. V. Diwan, Eur. J. Pharmacol., 2012, 678, 48 CrossRef CAS PubMed.
- Y. Lin, R. X. Shi, X. Wang and H. M. Shen, Curr. Cancer Drug Targets, 2008, 8, 634 CrossRef CAS.
- R. Liu, M. Gao, G. F. Qiang, T. T. Zhang, X. Lan, J. Ying and G. H. Du, Neuroscience, 2009, 162, 1232 CrossRef CAS PubMed.
- B. Xu, X. X. Li, G. R. He, J. J. Hu, X. Mu, S. Tian and G. H. Du, Eur. J. Pharmacol., 2010, 627, 99 CrossRef CAS PubMed.
- H. Yamamoto, J. Sakakibara, A. Nagatsu and K. Sekiya, J. Agric. Food Chem., 1998, 46, 862 CrossRef CAS.
- L. G. Gao, H. Wang, X. L. Song and W. Cao, J. Mol. Struct., 2013, 1034, 386 CrossRef CAS PubMed.
- A. Rygula, T. P. Wrobel, J. Szklarzewicz and M. Baranska, Vib. Spectrosc., 2013, 64, 21 CrossRef CAS PubMed.
- J. F. Li, L. S. Wang, H. Q. Bai, B. Yang and H. Yang, Med. Chem. Res., 2009, 18, 1 CrossRef CAS.
- G. K. B. Lopes, H. M. Schulman and M. Hermes-Lima, Biochim. Biophys. Acta, 1999, 1472, 142 CrossRef CAS.
- S. V. Jovanovic, S. Steenken, M. Tosic, B. Marjanovic and M. G. Simic, J. Am. Chem. Soc., 1994, 116, 4846 CrossRef CAS.
- A. Pękal, M. Biesaga and K. Pyrzynska, Biometals, 2011, 24, 41 CrossRef PubMed.
- (a) A. van den Bergen, R. Colton, M. Percy and B. O. West, Inorg. Chem., 1993, 32, 3408 CrossRef CAS; (b) G. Maccarrone, R. Caruso, A. Contino, A. Giuffrida, M. Messina and V. Cucinotta, Eur. J. Inorg. Chem., 2009, 2612 CrossRef CAS.
- W. Henderson and J. S. McIndoe, Mass Spectrometry of Inorganic, Coordination and Organometallic Compounds, John Wiley & Sons, Ltd, Chichester, 2005 Search PubMed.
- H. K. Kim, B. S. Cheon, Y. H. Kim, S. Y. Kim and H. P. Kim, Biochem. Pharmacol., 1999, 58, 759 CrossRef CAS.
- C. Hu and D. D. Kitts, Mol. Cell. Biochem., 2004, 265, 107 CrossRef CAS.
- D. loggia, E. Ragazzi and A. Tubaro, Pharmacol. Res. Commun., 1988, 20, 91 CrossRef.
- H. P. Kim, I. Mani and V. A. Ziboh, Prostaglandins, Leukotrienes Essent. Fatty Acids, 1988, 58, 17 CrossRef.
- J. G. Teixeira, C. B. Dias and D. M. Teixeira, Electroanalysis, 2009, 21, 2345 CrossRef CAS.
- B. Bozal, B. Uslu and S. A. Özkan, Int. J. Electrochem., 2011, 1 CrossRef PubMed.
- A. M. O. Brett and M. E. Ghica, Electroanalysis, 2003, 15, 1745 CrossRef CAS.
- A. L. Liu, S. B. Zhang, L. Y. Huang, Y. Y. Cao, H. Yao, W. Chen and X. H. Lin, Chem. Pharm. Bull., 2008, 58, 745 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra01396d |
| This journal is © The Royal Society of Chemistry 2014 |