A novel tunable Na2Ba6(Si2O7)(SiO4)2:Ce3+,Mn2+ phosphor with excellent thermal stability for white light emitting diodes
Wenzhen Lvab,
Yongchao Jiaab,
Qi Zhaoab,
Mengmeng Jiaoab,
Baiqi Shaoab,
Wei Lva and
Hongpeng You*a
aState Key Laboratory of Rare Earth Resource Utilization, Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, Changchun, 130022, P. R. China. E-mail: hpyou@ciac.ac.cn
bGraduate School of the Chinese Academy of Sciences, Beijing, 100049, P. R. China
First published on 28th February 2014
Abstract
In this paper, Ce3+ doped and Ce3+,Mn2+ co-doped Na2Ba6(Si2O7)(SiO4)2 phosphors were synthesized via a high temperature solid-state reaction. The Rietveld refinement analysis of the X-ray diffraction patterns confirmed the formation of the single phase of Na2Ba6(Si2O7)(SiO4)2. The PL spectrum of the Ce3+ single-doped phosphor shows a broad asymmetric band extending from 350 to 600 nm peaking at 420 nm under the excitation of UV light. Also, the low temperature (5 K) PL spectrum shows clearly three peaks at 375, 420, and 451 nm, which is in accordance with the Ce3+ ion site in the host. The Ce3+,Mn2+ co-doped phosphors show a blue emission band and an orange emission band, and the corresponding CIE coordinates intuitively indicate the tunable colors from the blue to green area. And the energy transfer mechanism from Ce3+ to Mn2+ in the host has been verified to be a dipole–quadrupole interaction. In addition, the emission intensity of Na2Ba6(Si2O7)(SiO4)2:0.01Ce3+ phosphor slightly changed with the temperature increase from 300 to 450 K, revealing that the obtained phosphor possesses an excellent thermal stability, which makes it an attractive candidate phosphor for white lighting emitting diodes.
1. Introduction
In the past decade, solid-state white light emission has confirmed its irreplaceable status for new solid-state light sources in the context of environmental and energy saving issues. As a result, phosphors, as an important part of white lighting emitting devices have aroused worldwide research interest.1–3 Phosphors are always composed of two indispensable compositions as the matrix and luminescence center. The matrix is always developed from the various compounds. Always, silicate with diverse, rigid and very stable structures have been investigated widely for either their physicochemical phenomena and mechanisms or available application and practical usage in phosphor.4 Among various silicate compounds, Sr3SiO5 has been highlighted and chosen as an matrix for many years, because the Sr2SiO5 doped with Ce3+ ion phosphor shows excellent performance as a yellow component for WLEDs.5,6 As to the luminescence center, we have to mention Ce3+ ion, which always acted as the luminescence centers in phosphor. The Ce3+ ion can be excited by either a UV chip or a blue chip. The emission band of Ce3+ ion is usually composed of a broad band and varies from ultraviolet to yellow light, depending on the host, which is caused by the Ce3+ ion 4f1 ground state undergoing parity allowed f–d transition with 5d excited state.7 Therefore, matrices doped with Ce3+ ions show great potential as phosphor for white lighting emitting diodes, and indeed receive a lot of attention recently. As a result, many useful phosphors have been reported successively.8,9 The famous Ce3+ doped yellow phosphor is Y3Al5O12:Ce3+, however, the phosphor suffers a low Ra when pumped by a blue LED chip, which is caused by the lack of thermal stability at elevated temperatures during white LED operation. Therefore, most researchers are committed on developing novel phosphors with improved thermal stability now. In addition, the Ce3+ ion, apart from its own intense emission, can facilitate the energy transfer to various luminescent centers such as Tb3+ ion or Mn2+ ion, which can significantly solve the overheating problem associated with inappropriate excitation for WLEDs.10,11Here, we choose Na2Ba6(Si2O7)(SiO4)2 matrix as a research objectives. Notwithstanding the fact that the Na2Ba6(Si2O7)(SiO4)2 matrix has been first reported by Tamazyan et al.,12 the most interesting feature of the co-existence structure of Si2O7 and SiO4 in the Na2Ba6(Si2O7)(SiO4)2 matrix has not attracted much attention from materials scientists until recently. Also, to our best knowledge, neither investigations regarding on the luminescence properties of Na2Ba6(Si2O7)(SiO4)2 nor the energy transfer mechanism from Ce3+ to Mn2+ in the host has been reported. Therefore, we speculate whether the unique structure doped with Ce3+ or Mn2+ can produce some surprising phenomenon. Here, we focus on investigating the crystal structure, the thermal stability and the luminescent properties of Ce3+ or Ce3+,Mn2+ co-doped Na2Ba6(Si2O7)(SiO4)2 phosphors. The result indicates that this novel developed phosphor has excellent thermal stability and shows tunable blue to yellow emission excited by UV light.
2. Experimental section
2.1 Materials synthesis
Polycrystalline powder samples with the compositions of Na2Ba6(Si2O7)(SiO4)2:xCe3+,yMn2+ (NBSO:Ce3+,Mn2+) were synthesized via a conventional solid-state reaction with NaNO3 (A.C), BaCO3 (A.C), SiO2 (A.C), CeO2 (99.99%) and MnCO3 (99.99%) as the raw materials. The stoichiometric amounts of the raw materials were weighed out and thoroughly mixed by grinding in an agate mortar. Afterward, the obtained product was then annealed at 1050 °C for 8 h with an intermediate regrinding under a 5% H2–95% N2 atmosphere. Finally, the temperature lowered to room temperature and some white polycrystalline powder was obtained.2.2 Characterization
The crystalline phases of the samples were identified by X-ray diffraction (XRD), which were performed on a D8 Focus diffractometer (Bruker) operating at 40 kV and 40 mA with Cu.Kα radiation. The X-ray diffraction data were recorded as follows: 2θ range: 15°–70°, scanning speed: 0.5 min, 0.02 step. The powder diffraction data were subjected to performa computer software General Structure Analysis System (GSAS) package.13 The photoluminescence (PL) and photoluminescence excitation (PLE) spectra of the obtained powders were recorded with a Hitachi F-4500 spectrophotometer equipped with a 150 W xenon lamp as the excitation source. Thermoluminescence (TL) spectra were measured with a three-dimensional (3D)-TSL spectra instrument. The luminescence decay curve was obtained from a Lecroy Wave Runner 6100 digital oscilloscope (1 GHz) using a tunable laser (pulse width = 4 ns, gate = 50 ns) as the excitation source (Continuum Sunlite OPO). All the measurements were performed at room temperature.
3. Results and discussion
3.1 Na2Ba6(Si2O7)(SiO4)2:Ce3+ phosphor
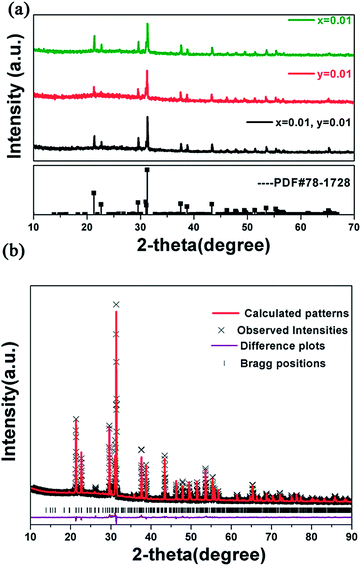
Fig. 1(a) shows the X-ray diffraction patterns of several representative Na2Ba6(Si2O7)(SiO4)2:0.01Ce3+,yMn2+ samples. It is obvious that all the diffraction peaks of the samples can be exactly indexed to the standard data of Na2Ba6(Si2O7)(SiO4)2 (JCPDS card no. 78-1728), which indicates that the prepared phosphors are of single phase and the doped Ce3+ and Mn2+ ions have been incorporated into the host successfully. To further investigate the structure of the Na2Ba6(Si2O7)(SiO4)2 host, Rietveld refinement of the X-ray diffraction patterns of the Na2Ba6(Si2O7)(SiO4)2:0.01Ce3+ sample has been done at room temperature as Fig. 1(b) shows.13 The initial structural model was constructed with crystallographic data previously reported for Na2Ba6(Si2O7)(SiO4)2 (JCPDS78-1728). The crystallographic cell parameters proceeded smoothly to convergence and do not show a significant change considering the standard deviations. All of the observed peaks satisfy the reflection condition, χ2 = 7.85, Rp = 6.28% and Rwp = 8.819%. The refinement result indicates the Na2Ba6(Si2O7)(SiO4)2 has space group P21/a with unit cell parameters a = 11.52 Å, b = 9.508 Å, c = 7.856 Å, V = 820.64 Å3, and Z = 2. Meanwhile, the refinement result further verifies that the structure of Na2Ba6(Si2O7)(SiO4)2 host is unchanged with the doping of luminescence ions.Fig. 2(a) and (b) represents a spatial view of the Na2Ba6(Si2O7)(SiO4)2 unit cell from different directions. One can see clearly the discrete [SiO4] anions (pink polohedron) and isolated [Si2O7] anions (gray polohedron) in this unit cell. The discrete [SiO4] anions and isolated [Si2O7] anions form unique layer in sandwich package way. The isolated [SiO4] anions arrange in the inside of the cell, while the isolated [Si2O7] anions occupy the upper and lower plane. The entire above package manner to delimit right coordination cavities occupied by Ba2+ and Na+ cations, respectively. There are three crystallographic independent Ba2+ ions positions denoted as Ba(1), Ba(2), and Ba(3), in which Ba(1) and Ba(2) are in 9-coordination environment with tricapped trigonal prismatic geometry, while Ba(3) occupies 10-coordinated O polyhedra constructing bicapped square prism which builds distorted polyhedron with oxygens interconnected via common edges, respectively. Fig. 2(c) displays the different coordination environments of the three Ba2+ ions. Another cation in the host, the Na+ ion is all coordinated by six oxygen atoms in the form of an octahedra. On the basis of the above structure analysis, the coordination diversity of the Ba2+ sites in the host is benefit to the luminescence.12
 | ||
| Fig. 2 Crystal structure of Na2Ba6(Si2O7)(SiO4)2 in different directions (a) and (b); coordination of three different Ba2+ ions (c). | ||
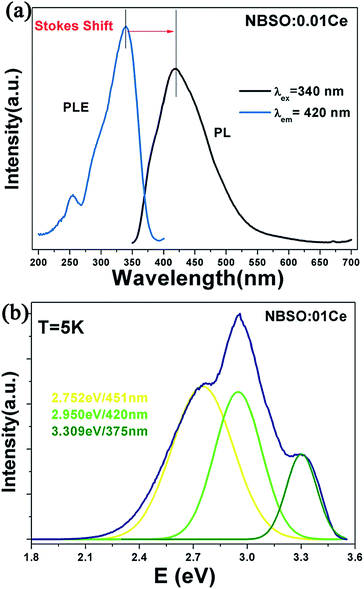
Fig. 3(a) shows the PLE and PL spectra of Na2Ba6(Si2O7)(SiO4)2:0.01Ce3+. The PLE spectrum is composed of a broad band range from 200 to 400 nm, a characteristic feature of the Ce3+ ions emission band from the 4f1 ground state to the 5d level as described by Judd–Ofelt theory. The PL spectrum shows a broad asymmetric band extending from 350 to 600 nm peaking at 420 nm.14,15 The asymmetry of PL band is always caused by the various luminescence sites in the host. Always, the value of Stokes shift is an important property of PL spectrum, which can measure the stiff of host lattice and the degree of the nonradiative relaxation after the luminescent ions excited. The Stokes shift can be roughly estimated as twice of the energy difference between the peak energy of the emission band and the zero-phonon line energy that was empirically determined as the intersection point of the excitation spectrum and emission spectrum.16 The Stokes shift of emission is roughly calculated to be 0.694 eV in energy here. In order to better understand the photoluminescence property of Ce3+ ion, we measure the PL spectrum of Na2Ba6(Si2O7)(SiO4)2:0.01Ce3+ at 5 K (Fig. 3(b)). The emission curve has been well-fitted with a sum of three Gaussian functions in energy. One can see clearly a broad band with three peaks at 375, 420 and 451 nm, respectively. These components can be ascribed to the contributions of the transitions from the lowest 5d excited states to the ground states in three different Ce3+ luminescence centres, which is in accord with the number of Ba2+ sites in the host. It has been suggested the d-band edge (E) in energy of Ce3+ ion emission is sensitive to electron–electron repulsion, which always obey an empirical relation by Van Uitert as following:17
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cm−1), E is the position for the Ce3+ ion emission peak, V is the valence of the Ce3+ ion (V = 3), n is the number of anions in the immediate shell about the Ce3+ ion, Ea is the electron affinity of the atoms (eV), and r is the radius of the host cation replaced by Ce3+ ion (Å). Ea is a constant in the same host. Here, V = 3, Q* = 50
000 cm−1), E is the position for the Ce3+ ion emission peak, V is the valence of the Ce3+ ion (V = 3), n is the number of anions in the immediate shell about the Ce3+ ion, Ea is the electron affinity of the atoms (eV), and r is the radius of the host cation replaced by Ce3+ ion (Å). Ea is a constant in the same host. Here, V = 3, Q* = 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cm−1, the value of E is directly proportional to the product of n and r. In our case, Ba(1) and Ba(2) sites are nine-coordinated by nine O atoms with Ba–O distance of 2.890 Å and 2.894 Å, respectively. The Ba(3) site is ten-coordinated with O atoms at an average Ba–O distance of 2.938 Å. Therefore, we can get a conclusion that the band centred at 375 nm is attributed to the 5d–4f emission of Ce3+ ion occupied the Ba(3) ion site with ten-coordinate, and the bands at 451 and 420 nm are due to the Ce3+ ion occupied Ba(1) and Ba(2) ion site with nine coordination.
000 cm−1, the value of E is directly proportional to the product of n and r. In our case, Ba(1) and Ba(2) sites are nine-coordinated by nine O atoms with Ba–O distance of 2.890 Å and 2.894 Å, respectively. The Ba(3) site is ten-coordinated with O atoms at an average Ba–O distance of 2.938 Å. Therefore, we can get a conclusion that the band centred at 375 nm is attributed to the 5d–4f emission of Ce3+ ion occupied the Ba(3) ion site with ten-coordinate, and the bands at 451 and 420 nm are due to the Ce3+ ion occupied Ba(1) and Ba(2) ion site with nine coordination.
 | ||
| Fig. 3 PLE spectrum observed at 420 nm (a); PL spectrum of Na2Ba6(Si2O7)(SiO4)2:0.01Ce3+ at room temperature and 5 K under excited at 330 nm (b). | ||
The thermal quenching property is an important technological parameter for phosphors used in practical solid-state lighting. Fig. 6 indicates the temperature-dependent relative emission intensities of the as-prepared Na2Ba6(Si2O7)(SiO4)2:0.01Ce3+ phosphor under the 340 nm excitation. With the temperature increasing from room temperature to 450 K, the integrated emission intensity of Na2Ba6(Si2O7)(SiO4)2:0.01Ce3+ phosphor changes from 100% (300 K) to 93.7% (450 K), indicating that the phosphor possesses a excellent thermal stability. To verify the origin of temperature dependent emission intensity IT, the activation energy ΔE (the electrons excited from 4f states to the lowest 5d states of Ce3+ ion) can be described as the following equation:18,19
 | ||
| Fig. 4 PL intensity of Na2Ba6(Si2O7)(SiO4)2:0.01Ce3+ from room temperature to 450 K (λex = 340 nm). The inset shows the plot ln[I0/IT − 1] versus 1/kT. | ||
3.2 Na2Ba6(Si2O7)(SiO4)2:Ce3+,Mn2+ phosphor
As shown in Fig. 5, the PL spectrum of Na2Ba6(Si2O7)(SiO4)2:0.01Ce3+ shows a broadband emission from 350 to 600 nm centered at 420 nm, which was attributed to the f–d transition. The PLE spectrum of Na2Ba6(Si2O7)(SiO4)2:0.03Mn2+ contains several bands centered at 344, 365, and 413 nm, corresponding to the transitions from the 6A1(6S) ground state to the 4E(4D), 4T2(4D), and [4A1(4G), 4E(4G)] excited states, respectively. The significant spectral overlap between the PL spectrum of Ce3+ and PLE spectrum of Mn2+ indicates that the energy level of Ce3+ ions matches with well the energy level of Mn2+ ions. Thus, we can expect an effective resonance-type energy transfer from the Ce3+ to Mn2+ ions. Moreover, as shown in Fig. 6, another possible evidence, the PLE spectrum monitoring the emission (585 nm) of the Mn2+ and that (420 nm) of Ce3+ are similar. Under 340 nm excitation, the PL spectrum of the codoped sample Na2Ba6(Si2O7)(SiO4)2:0.01Ce3+,0.01Mn2+ in Fig. 6 shows both a blue band corresponding to the f–d transition of Ce3+ ions and a yellow band attributing to the 4T1–6A1 transition of Mn2+ ions. As a result, we can get various color tunes via adjusting the Mn2+ ions content.23Fig. 7 depicts the PL spectra of several Na2Ba6(Si2O7)(SiO4)2:0.01Ce3+,yMn2+ (y = 0.01, 0.03, 0.07, 0.09, 0.11) phosphors. We can observe clearly that the PL luminescent intensity of Ce3+ ions decreases with the Mn2+ ions concentration up to 0.11. However, the emission intensity of Mn2+ ions shows an enhancement initially until 0.07, beyond which its intensity shows a drastic reduction. The phenomenon indeed indicate efficient energy transfer from Ce3+ to Mn2+ ions.
 | ||
| Fig. 7 PL spectra of Na2Ba6(Si2O7)(SiO4)2:0.01Ce3+,yMn2+ (y = 0.01, 0.03, 0.07, 0.09, 0.11) phosphors under 340 nm excitation. | ||
The energy transfer efficiency (ηT) from Ce3+ to Mn2+ ions in Na2Ba6(Si2O7)(SiO4)2:0.01Ce3+,yMn2+ can be calculated by the formula:24
where V is the volume of the unit cell, xc is the critical concentration of the activator ion, and N represents the number of sites that the Ce3+ ion can occupy in per unit cell. For the Na2Ba6(Si2O7)(SiO4)2 host, N = 12, xc = 0.07, and V = 820.64 Å3, Therefore, the Rsa value is calculated to be 12.3 Å. As we know, the resonant energy-transfer mechanism consists of two types: one is exchange interaction and another is multipolar interaction. It is known that if energy transfer takes the exchange interaction, the critical distance between the sensitizer and activator should be shorter than 3–4 Å. Here, the critical distance is longer than 3–4 Å, which indicates the more possibility of energy transfer via the multipolar interaction mechanism.
According to Dexter's energy transfer expressions of multipolar interaction, the following relation can be easily obtained:25,26
where fq is the oscillator strength of the involved absorption transition of the acceptor, λs (in Å) is the emission position of the sensitizer (in nm), R is the distance between the activator and acceptor, τS is the decay lifetime of the sensitizer (in seconds). E is the emission energy of the Ce3+ ion (in eV), and ∫Fs(E)Fa(E) dE/E4 represents the spectral overlap between the normalized shapes of the sensitizer emission and the acceptor excitation. The critical distance Rsa between the sensitizer and activator is defined as the distance at which the probability of energy transfer equals the probability of radiative emission of the Ce3+ ions. Namely, at the critical distance there has the equation Psaτs = 1. Then, Rsa can be obtained by the following formula:27,28
On the basis of the above equation combined with fq = 10−10, the critical distance RC was calculated to be 11.6 Å, which agrees approximately with that obtained by using the concentration-quenching method.
To determine the quantum efficiency of photoconversion for this phosphor. Herein we applied the integrated sphere method for the measurements of optical absorbance (A) and quantum efficiency (Φ) of the phosphors. The optical absorbance and quantum efficiency of Na2Ba6(Si2O7)(SiO4)2:Ce3+,Mn2+ phosphors were calculated by using the following two equations:
where L0(λ) is the integrated excitation profile when the sample is diffusely illuminated by the integrated sphere's surface, Li(λ) is the integrated excitation profile when the sample is directly excited by the incident beam, Ei(λ) is the integrated luminescence of the sample upon direct excitation, and E0(λ) is the integrated luminescence of the sample excited by indirect illumination from the sphere. The term Le(λ) is the integrated excitation profile obtained from the empty integrated sphere (without the sample present). On the basis of the above two equations, the measured values of quantum efficiency were 43.1%, 36.7%, 35.2%, 30.4%, 22.7%, 17.1% in Na2Ba6(Si2O7)(SiO4)2:0.01Ce3+,yMn2+ phosphor with y = 0, 0.03, 0.07, 0.09, 0.11 material at room temperature under the excitation wavelength of 340 nm. The higher quantum efficiency can be obtained by further improving the synthesis conditions to reduce the number of defects and impurities and to get a high crystallization of the phosphors.
Fig. 10 portrays the CIE chromaticity coordinates for the Na2Ba6(Si2O7)(SiO4)2:0.01Ce3+,yMn2+ (0.01, 0.03, 0.07, 0.09, 0.11) phosphors under 340 nm excitation. Generally, the coordinates shift from blue area (point A) to yellow area (point F) with the increase of Mn2+ ion concentration. It is found that the CIE chromatic coordinate is located at (0.348, 0.262) in the white-light area when the concentration of Mn2+ ions increases to y = 0.07. And the ideal CIE chromatic coordinate of WLEDs can be got by optimizing the amount of Ce3+ ions and Mn2+ addition simultaneously. The result indicates the novel Na2Ba6(Si2O7)(SiO4)2:Ce3+,Mn2+ with tunable color hue has a great potential phosphor fo UV-excited WLEDs.
 | ||
| Fig. 10 CIE chromaticity diagram Na2Ba6(Si2O7)(SiO4)2:0.01Ce3+,yMn2+ (0.01, 0.03, 0.07, 0.09, 0.11) phosphors under 340 nm excitation. | ||
4. Conclusion
In summary, we have synthesized novel Na2Ba6(Si2O7)(SiO4)2:Ce3+ and Na2Ba6(Si2O7)(SiO4)2:Ce3+,Mn2+ phosphors by high temperature solid state method. The Na2Ba6(Si2O7)(SiO4)2:Ce3+ phosphor show a broad emission band under UV excitation and excellent thermal stability. The Na2Ba6(Si2O7)(SiO4)2:Ce3+,Mn2+ phosphor shows tunable color by controlling the composition of Mn2+ content. The energy transfer from Ce3+ ion to Mn2+ ion has been confirmed to be via a dipole–quadrupole mechanism on the basis of the Dexter's energy transfer theory. The involved critical distance of energy transfer has also been calculated by concentration quenching method and spectral overlap method. All the above investigations show that the novel Na2Ba6(Si2O7)(SiO4)2:Ce3+,Mn2+ phosphor with tunable emission property has great potential as novel phosphor for UV-WLEDs.Acknowledgements
This work is financially supported by the National Natural Science Foundation of China (Grant no. 21271167) and the Fund for Creative Research Groups (Grant no. 21221061).References
- C. Feldmann, T. Justel, C. R. Ronda and P. J. Schmidt, Adv. Funct. Mater., 2003, 12, 511 CrossRef.
- C. C. Lin and R. S. Liu, J. Phys. Chem. Lett., 2011, 2, 1268–1277 CrossRef CAS.
- X. Li, J. D. Budai, F. Liu, J. Y. Howe, J. Zhang, X. Wang, Z. Gu, C. Sun, R. S. Meltzer and Z. Pan, Light: Sci. Appl., 2013, 2, e50 CrossRef.
- J. K. Park, M. A. Lim, C. H. Kim, H. D. Park, J. T. Park and S. Y. Choi, Appl. Phys. Lett., 2003, 82, 683 CrossRef CAS PubMed.
- H. S. Jang and D. Y. Jeon, Appl. Phys. Lett., 2007, 90, 041906 CrossRef PubMed.
- H. Luo, J. Liu, X. Zheng, L. Han, K. Ren and X. Yu, J. Mater. Chem., 2012, 22, 15887–15893 RSC.
- Z. Tao, Y. Huang and H. J. Seo, Dalton Trans., 2013, 42, 2121–2129 RSC.
- D. Haranath, H. Chander, P. Sharma and S. Singh, Appl. Phys. Lett., 2006, 89, 173118 CrossRef PubMed.
- Y. Q. Li, N. Hirosaki, R. J. Xie, T. Takeda and M. Mitomo, Chem. Mater., 2008, 20, 6704–6714 CrossRef CAS.
- C. Duan, Z. Zhang, S. Rosler, S. Rosler, A. Delsing, J. Zhao and H. T. Hintzen, Chem. Mater., 2011, 23, 1851–1861 CrossRef CAS.
- W. Lv, W. Lü, N. Guo, Y. Jia, Q. Zhao, M. Jiao, B. Shao and H. You, Dalton Trans., 2013, 42, 13071–13077 RSC.
- R. A. Tamazyan, A. Malinovskii and M. I. Sirota, Sov. Phys. Crystallogr., 1987, 32, 519–522 Search PubMed.
- A. C. Larson and R. B. Von Dreele, Los Alamos National Laboratory Report LAUR, 1994 Search PubMed.
- C. Liu, H. Liang, X. Kuang, J. Zhong, S. Sun and Y. Tao, Inorg. Chem., 2012, 51, 8802–8809 CrossRef CAS PubMed.
- Y. Jia, H. Qiao, Y. Zheng, N. Guo and H. You, Phys. Chem. Chem. Phys., 2012, 14, 3537–3542 RSC.
- G. Ju, Y. Hu, L. Chen and X. Wang, J. Appl. Phys., 2012, 111, 113508 CrossRef PubMed.
- L. G. Van Uitert, J. Lumin., 1984, 29, 1–9 CrossRef CAS.
- C. Huang, Y. Chen, T. Chen, T. Chan and H. Sheu, J. Mater. Chem., 2011, 21, 5645 RSC.
- J. Y. Han, W. B. Im, G. Lee and D. Y. Jeon, J. Mater. Chem., 2012, 22, 8793–8798 RSC.
- G. Lee, J. Y. Han, W. B. Im, S. H. Cheong and D. Y. Jeon, Inorg. Chem., 2012, 51, 20 Search PubMed.
- C. Liu, Z. Xia, Z. Lian, J. Zhou and Q. J. Yan, J. Mater. Chem. C, 2013, 1, 7139–7147 RSC.
- G. Blass, J. Chem. Phys., 1969, 51, 3529 CrossRef PubMed.
- L. Shi, Y. Huang and H. J. Seo, J. Phys. Chem. A, 2010, 114, 6927–6934 CrossRef CAS PubMed.
- P. I. Paulose, G. Jose, V. Thomas, N. V. Unnikrishnana and M. K. R. Warrier, J. Phys. Chem. Solids, 2003, 64, 841–846 CrossRef CAS.
- W. Yang, L. Luo, T. Chen and N. Wang, Chem. Mater., 2005, 17, 3883–3888 CrossRef CAS.
- G. Blasse, Philips Res. Rep., 1969, 24, 131 CAS.
- G. Li, D. Geng, M. Shang, Y. Zhang, C. Peng, Z. Cheng and J. Lin, J. Phys. Chem. C, 2011, 115, 21882–21892 CAS.
- H. You, J. Zhang, G. Hong and H. Zhang, J. Phys. Chem. C, 2007, 111, 10657–10661 CAS.
| This journal is © The Royal Society of Chemistry 2014 |