A simple and eco-sustainable method for the sulfonylation of amines under microwave-assisted solvent-free conditions†
Abstract
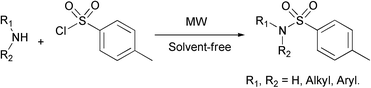
A new environmentally benign, simple, and efficient protocol for the chemoselective sulfonylation of various structural amines using microwave irradiation under solvent- and catalyst-free conditions is reported. The corresponding sulfonamides were obtained in excellent yields within short reaction times. Simplicity, milder, cleaner and greener conditions, easier work-up, and lower generation of waste or pollution are the main advantages of this method.

 Please wait while we load your content...
Please wait while we load your content...