Rational design of drug-eluting stents via electrospray and in vivo evaluation of preventing oesophageal stricture†
Lili
Zhao‡
a,
Ying
Gao‡
b,
Guoxiang
Gu
a,
Jinhui
Wu
*a,
Zhining
Fan
b,
Hong
Dong
a and
Yiqiao
Hu
*a
aState Key Laboratory of Pharmaceutical Biotechnology, Nanjing University, Nanjing 210093, P.R. China. E-mail: huyiqiao@nju.edu.cn; wuj@nju.edu.cn
bInstitute of Digestive Endoscopy and Medical Center for Digestive Diseases, Second Affiliated Hospital of Nanjing Medical University, Nanjing 210011, P.R. China
First published on 14th March 2014
Abstract
Stent-induced restenosis limits the clinical use of stents in the palliative treatment of oesophageal stricture. To prevent such secondary tissue hyperplasia, novel paclitaxel-coated stents were developed using electrospray in this study. Automatic electrospray could achieve precise control of formulation and coating pattern. After preparation, polyurethane film still maintained great mechanical properties at high drug-loading (≥20%), with maximum 6-fold elongation and over 20 MPa tear strength. Furthermore, drug-loading film kept the intactness and tight attachment with the stent throughout the experiment. Drug-release kinetics was similar between in vitro and in vivo tests. Approximately 50% paclitaxel was released in the early stage, followed by a sustained release that could maintain the local therapeutic concentration for 6 weeks or more. Rabbit was chosen as the animal model for the in vivo evaluation of preventing oesophageal stricture. Compared with non-drug coating stents, paclitaxel-eluting stents were safe and effective against tissue hyperplasia secondary to stent deployment. These results suggest that this paclitaxel-eluting stent and versatile electrospray technique would provide a promising strategy in the management of malignant and refractory benign oesophageal stricture.
Introduction
Clinically, stents have been widely used to palliate oesophageal stricture caused by benign or malignant conditions.1 Refractory benign obstruction has high recurrent rates and is associated with poor quality of life. For late-stage oesophageal cancer, half of patients suffer server dysphagia, and their 5 year survival rate is less than 15%.2,3 Studies showed stent deployment could rapidly relieve dysphagia with few complications. Commercial stents may be categorized as self-expandable metal (SEMS), self-expandable plastic (SEPS) and biodegradable stents. Among them, SEMS are recommended for clinical treatment because of their great efficacy and safety.4 However, tissue embedment and stent-related restenosis are still the major challenges for their routine use.1Restenosis is caused by tissue hyperplasia secondary to stent placement, such as benign fibrosis or malignant ingrowth. Systemic chemotherapy has limited benefit due to low local distribution. In situ drug delivery via a stent could be a potential strategy for overcoming this problem. Drug-eluting stents (DES) have been developed and widely used in the cardiovascular field.5 For example, TAXUS™ (paclitaxel) and CYPHER™ (rapamycin) are effective in relieving vascular obstruction and preventing post-procedural restenosis. Recent studies demonstrated that this concept (DES) has great potential in gastroenterology.6–8 However, clinical translation is still rare, partially due to the imperfect design of the drug-eluting stent. Jeon et al. had to employ surgical fixation for externally coated stents, which led to infection and high mortality.9 There are few studies focusing on the rational design, preparation and evaluation of drug-loading stents.
To develop the promising DES system for oesophageal stricture, critical concerns include coating material, drug, and preparation technique. Coating material is critical for adapting the complicated oesophageal environment and the long-time treatment. Polyurethanes (PUs) are synthetic polymers composed of two building blocks connected by a carbamate bond. Due to varied combinations of building blocks, PUs can be tailored to have various material properties, such as excellent biochemical stability, abrasion resistance and mechanical properties (extensibility and restorability).10 Just like other biomaterials,11 PUs are widely recommended for biomedical purposes.10,12 They have been already commercialized for artificial tissue, bandages, and condoms. Recent studies suggested PUs are also great carriers for sustained delivery of chemical drugs and biotherapeutics.13 These unique advantages make PUs eligible for drug-eluting stents.
Paclitaxel has excellent efficacy and versatile activities in vivo. As an antineoplastic agent, it is widely used as the first-line treatment for various cancers.14 It takes effect by interrupting the polymerization/de-polymerization of microtubules, which would trigger the apoptotic pathway. Paclitaxel could also have anti-proliferation and anti-angiogenesis effects. The anti-restenosis results are promising in the cardiovascular field. Therefore, paclitaxel-eluting stents might efficiently inhibit oesophageal stricture and hyperplasia caused by a variety of conditions. More importantly, the level of paclitaxel in the plasma after local delivery was shown to be about 1000-fold less than that after systemic administration,15,16 which would be helpful for maximizing therapeutic effect and minimizing systemic side-effect.
For the study reported here, electrospray was introduced to intelligently coat stents. This technique has been widely used for surface coating of various apparatuses.17 Similarly with the electrospinning technique,18 the convenience and reliability of electrospray has been confirmed for applications ranging in size from microns to meters.19,20 Using bio-inert polyurethanes, the current study utilized a tailor-developed electrospray system to prepare an internal coating of paclitaxel-eluting stents with controlled formulation, pattern and properties, which might facilitate transition from bench to bedside. Mechanical properties and drug-release behaviour were systematically characterized in vitro and in vivo. The efficacy and safety were investigated by comparing paclitaxel-eluting SEMS and non-DES in a rabbit model.
Materials and methods
Materials
Polyurethane was provided by Changzhou Garson Medical Stent Apparatus Ltd. Self-expandable Nitinol stents were self-designed and customized by Garson. Paclitaxel was purchased from Hongdoushan Co. Ltd (Jiangsu, China). All other chemicals were of analytical grade and used as received.Stent preparation
Stents (10 mm × 15 mm) were coated by electrospray of polyurethane mixed solution with/without drugs. The electrospray system was self-designed by referring to previous studies. The preparation procedure was as follows: polyurethane beads were first dissolved in a solvent mixture of tetrahydrofuran (THF) and N,N-dimethylactamide (DMF) (v/v 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). According to the drug loading requirement, paclitaxel was accurately weighed and added to stock polymer solution.
1). According to the drug loading requirement, paclitaxel was accurately weighed and added to stock polymer solution.
Each stent was laid on a clean glass tube with a diameter of approximately 10 mm. In our electrospray system, process parameters were optimized for the coating requirement. Solution was diluted to a final concentration of 30 mg ml−1. The syringe pump speed was 100 μl min−1, while the electric field intensity was 1.7 kv cm−1. The revolving speed was fixed at 100 rpm for the supporting drum. Tetrahydrofuran was quickly evaporated during the spraying process. Little DMF would remain and dry slowly under infrared light, which led to the formation of smooth and integrated films on the stents. The coated stent was then separated from the glass and dried overnight in an oven at 60 °C. After elimination of solvents, paclitaxel was evenly dispersed inside the film matrix. Products were stored in a desiccator before use.
Characteristics of drug-loaded film
The integrity and tractility of the polyurethane film was tested by Material Tension Tester (Instron, USA). First, aforementioned polymer solution (30 mg ml−1) was prepared with different drug loading (0%, 10%, 20%). One ml of solution was transferred into separate glass dishes. A triple sample was made for each formulation. The samples would be dried for 1 h under near-infrared light, and then for 2 days at 60 °C. Different loading films were cut into the same shape (1 cm × 4 cm). All samples were evaluated under consistent conditions. Film thickness was confirmed using a micrometer calliper. Detailed parameters were 10 mm min−1 speed and constant 1 kN load for distraction.An S-3400N II scanning electron microscope (SEM) (Hitachi, Japan) was utilized to observe surface characteristics of drug-loading films, such as smoothness, attachment and drug dispersal. Different from tension tests, all SEM samples are stents internally coated by electrospray. Varied formulations were tested, with different drug loading or polymer weight. And after drug release in vitro, the surface changes were also detected using SEM.
Drug release study
For in vitro release, drug-coating stents were immersed into a 25 ml conical flask with 10 ml of 0.1% SDS pH 6.5 PBS. The flask was kept in a shaking water bath, at 37 °C with a 200 rpm shaking speed. At predetermined time points, stents were transferred into fresh release buffer to maintain sink condition.The in vivo release profile was calculated by residual drug-loading on the stent. At different time points, drug-loading stents were removed after the rabbit was sacrificed. They were kept overnight in a 60 °C oven. Then, 10 ml THF was used to dissolve the polyurethane film on the stents. The THF solution was slowly dropped into 60 ml acetonitrile and diluted into a volume of 100 ml.
The amount of paclitaxel was analysed by HPLC (Shimadzu LC-10 system, Japan). An Agilent C-18 column (250 × 4.6 mm–5 μm) was used for the analysis. The mobile phase consisted of water and acetonitrile (v/v 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60). All samples were detected at 1 ml min−1 flow rate and 227 nm wavelength. All samples were tested in triplicate.
60). All samples were detected at 1 ml min−1 flow rate and 227 nm wavelength. All samples were tested in triplicate.
Animal study
All experiments were approved by the Institutional Animal Care and Use Committee in Nanjing University. New Zealand rabbits (2.8–3 kg) were purchased from Jinling Rabbit Farm. They were randomized into two group (10 per group): group A, polyurethane-coated stent without drug (non-DES); group B, paclitaxel-eluting stent (10% loading) (paclitaxel-DES). After stent placement, rabbits were sacrificed every two weeks. There were three rabbits in each group at every point. All rabbits were free to eat and to drink water.Stent deployment
Before placement, food was withheld from the rabbits for 12 hours. Anesthesia was induced by intramuscular administration of 1.5 mg kg−1 ketamine hydrochloride and 0.1 ml kg−1 of 2% xylazine per rabbit. Each drug-loaded stent was first fixed on an oesophageal stent introducer set. Under X-ray guidance, a guide wire was inserted into the stomach. Over the wire, the stent introducer set was passed into the oesophagus. According to X-ray imaging, each stent was deployed near the T5 vertebral body. Esophagography was also recorded to verify the performance of the stent in the oesophagus.Gross and microscopic examination
At pre-set points, rabbits were sacrificed by using an overdose of xylazine hydrochloride. Gross examination and tissue H & E staining were performed for both groups. The section of oesophagus around stents was surgically removed for each sample. Gross examination included mucosal nodularity, oesophageal perforation and other visible tissue changes. After removal of stents, the oesophageal specimen was fixed overnight in 10% buffered formalin solution. H & E staining was performed according to a previous protocol. Microscopic examination was carried out to evaluate the difference between paclitaxel-DES and non-DES groups, such as granulation tissue, sub-mucosal fibrosis and inflammatory cell infiltration. All samples were observed using a microscope (Zeiss, Germany).Results
Preparation of drug-coated stent
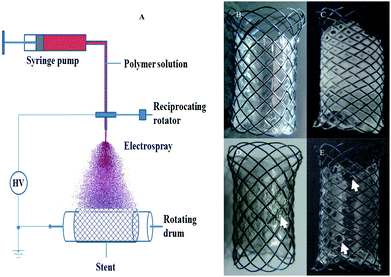
Modified from previous studies,21 a simple electrospray system was designed for controlling precise stent coating (Fig. 1A). The syringe pump would precisely control solution supply during the preparation. Total loading amount could be pre-set by adjusting solution concentration, the feeding speed and time. At the tip of the spray nozzle, the high voltage source would cause the polymer solution to carry electric charge. Inside the electric field, solution drops would repel each other and split into micro-range, which led to formation of a conical aerosol. Then micro-drops would move along the electric field gradient and deposit on bare nitinol stents. During the spray process, most of the THF would evaporate before forming a sediment. The remaining DMF-polymer drops would fuse together on the supporting surface and slowly dry to develop a uniform film. The reciprocating rotator allows the spray nozzle to move back and forth, which ensures uniform coating during production. In our experiment, the reciprocating part was removed due to the small size of the stent (10 × 15 mm). Stents were fully surrounded by the aerosol. The THF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMF (v/v 9
DMF (v/v 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) mixture is excellent for polyurethane electrospray coating. During preparation, a higher level of THF led to excessive evaporation and incomplete fusion (Fig. 2C). The film became opaque and fragile in this case. A high ratio of DMF would delay the whole process owing to slow evaporation. Based on stent size, the main parameters were optimized as follows: 30 mg ml−1 polymer solution, 100 μl min−1 feeding speed, 1.7 kv cm−1 field intensity and 100 rpm for rotating drum. Fig. 1B showed the perfect coating on the stent with 30 mg polyurethane. As shown in Fig. 1E, 15 mg of film usually contains holes, while, 100 mg polyurethane takes too long to prepare. Also, in this case, the film was too thick, stretch resistant, and was found to contain bubbles (Fig. 1D). Therefore, 30 mg stent coating was chosen for further investigations. There was no coating in flange-ends for hindering migration in normal rabbits.
1) mixture is excellent for polyurethane electrospray coating. During preparation, a higher level of THF led to excessive evaporation and incomplete fusion (Fig. 2C). The film became opaque and fragile in this case. A high ratio of DMF would delay the whole process owing to slow evaporation. Based on stent size, the main parameters were optimized as follows: 30 mg ml−1 polymer solution, 100 μl min−1 feeding speed, 1.7 kv cm−1 field intensity and 100 rpm for rotating drum. Fig. 1B showed the perfect coating on the stent with 30 mg polyurethane. As shown in Fig. 1E, 15 mg of film usually contains holes, while, 100 mg polyurethane takes too long to prepare. Also, in this case, the film was too thick, stretch resistant, and was found to contain bubbles (Fig. 1D). Therefore, 30 mg stent coating was chosen for further investigations. There was no coating in flange-ends for hindering migration in normal rabbits.
Characteristics of stent coating
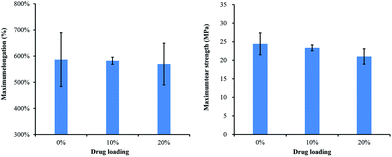
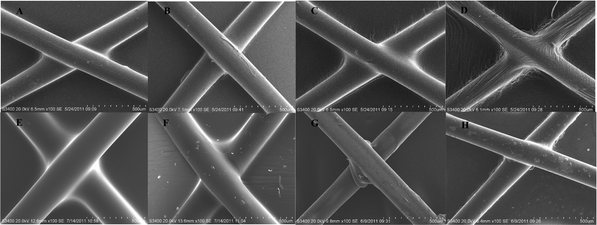
The physical properties of coating film, such as ductility and integrity, are critical for stents' deployment and long-term treatment. As shown in Fig. 2, with increasing drug loading from 0% to 20%, there are no obvious changes for maximum elongation. All samples could be stretched for almost six times their original lengths before being torn. During the experiment, samples could recover from a less than 3-fold stretch. These were also verified by manual pull-off tests of coated stents for which no visible deformation was observed. Fig. 2B shows that all test samples had strong tear strength of more than 20 Mpa. An increase of drug loading was associated with a slight decrease in the strength: the strength of the 20% group was decreased by about 3 Mpa.SEM was utilized to check smoothness of drug-loading film and its attachment on stents. In Fig. 3, panels A, C, E, and G represent, respectively, 30 mg-free drug, 15 mg-10% loading, 30 mg-10% loading, 30 mg-20% loading. For all samples, no drug aggregation was observed on the film surface, which indicates the high paclitaxel-dispersibility after electrospray preparation. After 5 days release, each group was examined again, correspondingly displayed in panels B, D, F, and H. As shown in Fig. 1, 15 mg polymer is not enough for stent coating. Fig. 3C demonstrates the loose connection between film and stent strut., such a disruptive structure occurred especially after drug release, as shown in Fig. 3D. Correspondingly, 30 mg polymer retained unbroken coverage on the stent after 5 days drug release and water infiltration. For 20% loading, drug loss induced partial rupture around the connecting corners. However, the entire film remained intact and smooth. During the preparation, drug-loading films were hard to separate from glass supports compared with those for which drugs were not loaded. SEM demonstrated that in adjoining sections, there were some wrinkles for the non-drug group, while drug-loading film smoothly wrapped stent struts.
In vitro sustained release
Paclitaxel is rarely soluble in water, about 0.3–3 μg ml−1 depending on crystal forms. For in vivo application, paclitaxel has high binding affinity with serum or intracellular proteins, which would greatly affect release behaviour of drug-loading stents. For the oesophagus, it's very hard to mimic the local environment, which includes oesophageal tissue, food, digestive fluids, etc. In our experiment, 0.1% SDS was used to improve paclitaxel solubility in pH 6.8 PBS release buffer, the paclitaxel release profile was evaluated for various DES formulations. In Fig. 4A, the drug-loading amount had a limited effect on its releasing kinetics. Similar accumulative–release profiles were obtained for drug loading ranging from 1% to 20%. Most of the drugs were released within 50 h of incubation. There was no significant difference among different groups. These results indicate that increasing loading could achieve predictably and efficiently desired drug concentrations locally. Before 10 h, all groups demonstrated burst-release patterns for about 50% drug. The effect of polyurethane content was also studied, as shown in Fig. 4B. While keeping the amount of paclitaxel constant (at 3 mg per stent), increased polyurethane content induces thicker film layers and slower release. Below 45 mg, polymer weight had limited effect on drug release. In 60 mg-PU samples, thicker film decreased drug density and increased the diffusion path, which led to slow release rate. The drug release might be controlled by a matrix-based pattern.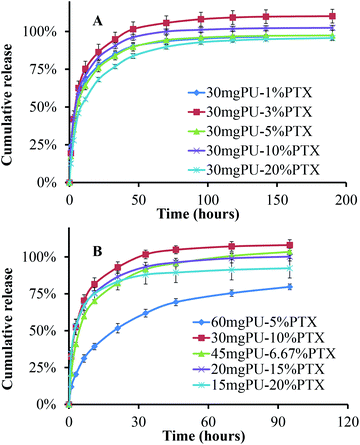 | ||
| Fig. 4 In vitro release of paclitaxel-eluting stent in 0.2% SDS-pH 6.8 PBS. (A) 30 mg polyurethane with different loading; (2) 3 mg paclitaxel with different polymer weight (n = 3). | ||
In vivo performance of stents
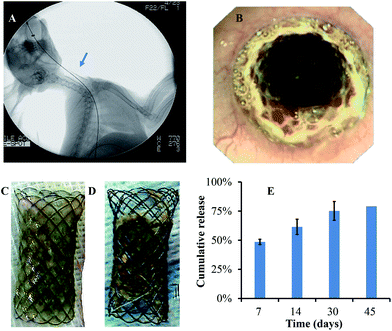
Beside suitable stent design, successful stent deployment is another important factor for application. Due to shortness of the oesophageal obstruction model, normal rabbits were chosen to observe in vivo the effect of the paclitaxel-coated stent. In our pre-experiments, a wrong position led to pain and death of rabbits. Stent migration occurred more often. After referring to previous research and experimental practice,8,22 the stent was finally placed in the distal portion of the oesophagus, near the T5 vertebral body (Fig. 5A).After stent placement, esophagography and follow-up inspection were performed. According to live imaging (Fig. 5B), the stent completely expanded and fitted well with oesophageal tissue. Daily diet, weight and behaviour were checked every other day. In the first few days, all rabbits suffered from stents and surgery, which led to less food consumption and weight loss. They all recovered after 1 week. During the following period of animal care, no obstruction and significant weight loss happened in either group.
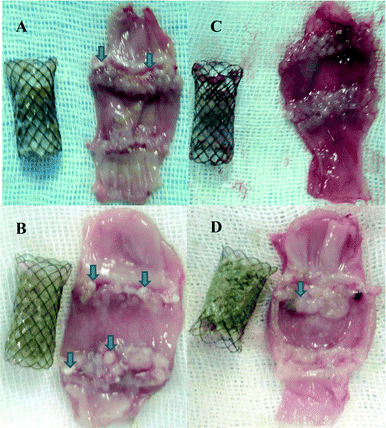
At predetermined time points (every two weeks), three rabbits were sacrificed for each group. After 45 days, there were obvious differences in the appearance of the stents between non-DES and paclitaxel-DES. Without drugs (Fig. 5C), stents were sticky and hard to separate from oesophageal tissue. There was lots of remaining oesophageal mucus on it and no visible boundary for polymer coating. In comparison, drug-loaded stents were clear (Fig. 5D). The polymer-coated film was covered with remains of ingested food. No tissue or debris remained on the distal ends. After separation, no visual damage was observed for film from either type of stent. All films showed high integrity, durability and reliability for a long time in in vivo applications, which is critical for stenosis or restenosis prevention. Meanwhile, calculated from remaining drug on stents, Fig. 5E shows an example of highly sustained in vivo release. The release profile was similar to in vitro results. About half of the original amount quickly dispersed into tissue in the first week. Then, the rate of drug efflux slowed down. After 45 days, there was about 20% paclitaxel retained in the stent. For this group, one of the three rabbits lost the stent due to migration. Inferring from the accelerated release profile of DES in vitro, stent would constantly release and keep local drug concentration for a long time after 80% release. Therefore, our product is supposed to be sustainably effective for more than 2 months.
Macro-pathologic examination
After sacrifice, all stents and oesophageal tissue were examined. No oesophageal perforation and stent destruction were observed. With the film's protection, almost no obvious nodularity could be found underneath the stent coating in all experimental groups. But in distal flange-sections, there were distinct differences in appearance between the two groups, which became more remarkable with time. After just a 2 week placement of non-DES, the distal strut of the stent was already entrapped inside the tissue, along with tissue ulcers. In comparison, there was some mild hyperplasia around bare struts of paclitaxel-DES (Fig. 6C). As shown in Fig. 6B, there were whitish tissue debris, and severe nodular hyperplasia after 4 week placement of the non-DES stent. In contrast, paclitaxel showed a great inhibition effect for oesophageal tissue granulation. The tissue specimen had a relatively smooth surface and moderate hyperplasia after 4 weeks (Fig. 6D). In addition, as shown in Fig. 6C (2 week), haemorrhagic tendency always occurred during the dissection of the oesophagus in early-stage experiments. Blood clots formed during this stage, as shown in Fig. 6D.Histopathologic examination
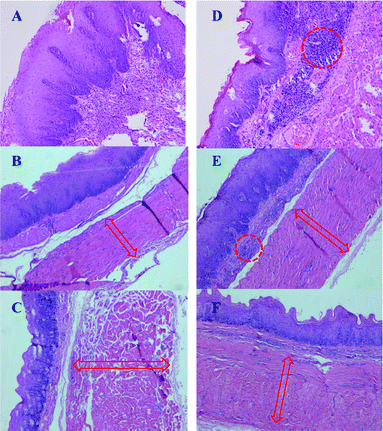
For oesophageal specimens, histopathology was performed to validate the mechanism of anti-hyperplasia and therapeutic safety. No radial perforation occurred for any sample. Fig. 7A, B and C show tissue samples of the non-DES group at the 2nd, 4th, and 6th week, respectively, while Fig. 7D, E and F show such samples for the paclitaxel-DES group. Unlike at the distal ends, there was merely fibrosis in middle parts of the tissue for all groups, which was also confirmed by macro-pathologic results. All samples are visualized at the same magnification in the figure. Compared with the 2 week specimen, later samples become dramatically thinner. Also, the muscular layer seemed to become much thicker and stronger with time. All of these changes might normally be the adaptive reaction for stents' dilatation force. However, sustained paclitaxel release still induced remarkable inflammation. As shown in Fig. 7D, the 2 week sample had lots of lymphocyte infiltration under the oesophageal mucosa. Meanwhile, notable vasodilatation and haemocyte infiltration occurred, which might explain macro-pathologic results about bleeding and blot. As time went on, the drug effect receded gradually but still worked until 6 weeks. All these indicated that as an in-situ drug delivery system, paclitaxel-DES are safe and effective for long-term treatment.Discussion
Both benign and malignant stricture of the oesophagus could induce dysphagia, malnutrition, weight loss or aspiration pneumonia and seriously affect patients' quality of life.23 In China, oesophageal cancer is the fifth most common malignant disease with high mortality.3 Unfortunately, complex oesophageal obstruction is associated with high recurrence rates and low response for regular treatment.1,23,24 With advances in clinical practice, novel self-expandable stents are gradually being accepted and recommended as the alternative strategy for refractory stenosis of the oesophagus. Instead of surgery accompanied by high morbidity and mortality, placement of stents is safe, cost-effective and minimally invasive for rapidly relieving clinical symptoms.4,25However, secondary restenosis often occurs as a delayed complication after deployment. The main cause is tissue hyperplasia around stent struts.26 As shown in some long-term studies of benign stricture, the incidence rate was up to 60% for bare stents and 13% for covered stents.8 Combined with migration, Song et al. showed almost 100% occurrence (12 of 12 patients) of delayed complication for long-term observation.27 As for long-term expectations of the treatment, these novel stents should yield significant improvements with regards to migration, anti-proliferation and retrieval.
In the past decade, drug-eluting stents have been developed and clinically used in the cardiovascular field. Sustained release of anti-proliferative drugs could efficiently inhibit vascular intimal hyperplasia and prevent secondary restenosis.28 Compared with systemic administration, in situ delivery would lower drug dosage, maximize local drug concentration and minimize systemic toxicity.29 Recent studies proposed that drug-eluting stents could also benefit other types of luminal stricture, ex. gastrointestinal tract, biliary.6–8
Conventional methods for oesophageal stent coating include film-precast and dip-drying.6,8,9 A prefabricated polymer film could externally wrap the stent and provide a well-defined formulation for the therapeutic requirement. But additional procedures must be adopted to prevent stent migration, such as surgical fixation or endoscopic clipping. These might lead to serious post-procedural complication, including infection, retching and high mortality. The other method, dip-drying, could internally coat the stent, but it's hard to precisely control and adjust the coating formulation. For post-market application, controllability of preparation methods and consistency of product quality are critical. For oesophageal obstruction, individualized stents have been highly recommended because of varied symptoms, such as tissue size, physiological location, and clinical stage of oesophageal stricture.4,23 Therefore, rational design, controlled preparation and customizability are needed for successful clinical translation of oesophageal drug-eluting stents.
In our studies, a tailor-made electrospray system was utilized to intelligently make stent coating. This technique could provide a convenient and reliable approach to prepare in situ film on stents.19,20 According to the adjustment of operating parameters, this automatic system could be customized to meet various requirements, for example, coating pattern (partially, fully or strut only covered) (ESI Fig. S5†), precise loading amount, specific size or shape of stents and so on. Our results showed that all final products looked smooth and had uniform mass and drug loading (ESI Table S1†). Furthermore, combined with distinct biomaterials and active drugs, this platform could create diverse drug-eluting stents for adapting to varied diseased conditions of luminal stricture. The excellent expansibility, stability and controllability would also benefit the future large-scale production.
Self-expandable nitinol stents were customized from Garson, with 10 mm × 15 mm knitted mesh structure. The 10 mm diameter was verified in previous rabbit experiments, which could minimize stent migration and post-procedural complication. Internal coating design would also allow protruding struts to slightly embed inside the mucosal layer and provide great support for stent fixation. An in situ cast would form a lattice-like construction, which would provide good maintenance for film integrity during long-term treatment. After 45 days placement in vivo, no visible breakage was found during our tests. Flange-shape ends were chosen for improving stent fixation in the normal rabbits. The bare metal structure induced mechanical injury and local tissue hyperplasia, which was used to observe the therapeutic effect of sustained paclitaxel.
In this study, paclitaxel-DES was designed and made based on the research above. With excellent polyurethanes, drug loading and release have a limited effect on the intrinsic structure and mechanical characteristics of films. This might be due to the micro-phase separation of the molecular building blocks in the structure, which endow PUs with more flexibility and elasticity.30,31 As shown in Fig. 3, paclitaxel would be uniformly dispersed throughout the film during the spray and cast. X-ray diffraction confirmed paclitaxel was amorphous in the final products (Fig. S3†). As indicated in SEM, hydrophobic paclitaxel might partially enhance intermolecular interactions, which led to tight attachment between membrane and supporting materials (nitinol strut or glass tube). Therefore, intact film provided favourable support and prevented tissue ingrowth underneath for 6 week treatment in rabbits. In vitro release suggested paclitaxel diffusion from PUs could be predictable and consistent within a certain range of formulation. From 15 to 45 mg polyurethane, content deviation was allowable and had limited effect on paclitaxel behaviour (Fig. 4B), which would reduce the requirement for precise formulation and greatly benefit large-scale production. Meanwhile, due to the similar release profile of different paclitaxel-loading DES, increasing stent loading would raise local drug concentration accordingly (Fig. 4A). Therefore, based on patients' condition, customized dosage could be designed and foreseen to maximize the therapeutic effect. In vivo results also confirm this dual-phase release pattern (Fig. 5E). The burst release would induce a pulse-therapy effect, which would rapidly reach local therapeutic concentration. Subsequently, sustained release would maintain local dosage for long-acting treatment. Paclitaxel usually takes effect in a dose-dependent and time-dependent manner.14–16 Most importantly, paclitaxel-eluting stents could be easily separated from the embedded tissue (Fig. 6), which would allow endoscopy-based replacement of DES for long-term treatment strategy. All these would greatly exert therapeutic efficacy.
Gross and microscopic examination confirmed paclitaxel-DES's therapeutic potential. During the surgery, it was hard to detach non-DES because of embedment into adjacent tissue after 2 or 4 weeks, while paclitaxel-DES has clear distal ends. And non-DES groups had more severe tissue nodularity. Probably, paclitaxel effectively inhibited re-epithelialization, granulation tissue formation and fibrosis. Significant increase of inflammatory cell infiltration occurred in drug groups. Paclitaxel might act by delaying and inhibiting inflammatory effects, while stents act as the continuous stimulus to recruit lymphocyte cell. The efficacy was also verified by only strut-coating stent for the in vivo test (ESI Fig. S5†). And compared with 1 μg mm−2 dose of commercial TAXUS,32 over 6 μg mm−2 paclitaxel of 10% loading stent was still safe and didn't induce any tissue perforation or other relative complication in the period of treatment.
Besides the encouraging results, further studies are still required. First, normal animal was not suitable for the evaluation of stricture treatment. In our study, partially-covered stent has to be used for preventing migration and inducing secondary tissue hyperplasia. As indicated in Fig. S5,† such hyperplasia could be prevented by improved DES. Ideally, fully-covered stents and rabbit primary stricture model would be studied to confirm the results described above. Then, detailed experiments should be performed to validate the dose response of different drug loadings, including pharmacokinetics, pharmacodynamics, pathologic changes and efficacy of longer treatment. Further, electrospray would be introduced to design more novel DES for complex luminal disease, ex. oesophageal hernia, and high trachea-oesophageal fistula.
Conclusion
For treatment of oesophageal stricture and stent-related restenosis, electrospray is introduced to make rational paclitaxel-eluting stents with internal coating. This novel automatic system provided a precise and convenient approach to manage product pattern, formulation and preparation. With bio-inert polyurethanes, paclitaxel-DES showed excellent mechanical properties in a controllable drug-release manner. These features would be favourable for individualized design to address the distinct conditions of each patient. As suggested by a 6 week experiment in vivo in the normal rabbits, paclitaxel-DES is safe and effective in reducing tissue hyperplasia secondary to stent deployment. Therefore, this novel DES design and versatile electrospray technique may provide an attractive strategy in the management of malignant and refractory benign oesophageal stricture.Acknowledgements
This research was supported by the Science and Technology Support Program of Jiangsu Province (no. BE2010719), the Natural Science Foundation of Jiangsu (no. BK2011572, BK2011859 and BK2011539), the National Natural Science Foundation (no. 81273464, 81172266, 81202474 and 30973651), the Research Fund for the Doctoral Program of Higher Education of China (no. 20110091120044), the Changzhou Special Project of Biotechnology and Bio-pharmacy (no. CE20105006), Jiangsu Innovation of Medical Team and Leading Talents Cultivation (no. LJ201127), and the Postdoctoral Foundation (no. 2012M521051).Notes and references
- P. Hindy, J. Hong, Y. Lam-Tsai and F. Gress, Gastroenterol. Hepatol., 2012, 8, 526–534 Search PubMed.
- M. Sundelöf, W. Ye, P. W. Dickman and J. Lagergren, Int. J. Cancer, 2002, 99, 751–754 CrossRef PubMed.
- Y. Lin, Y. Totsuka, Y. He, S. Kikuchi, Y. Qiao, J. Ueda, W. Wei, M. Inoue and H. Tanaka, J. Epidemiol., 2013, 23, 233–242 CrossRef.
- F. Zhang, G. Ji and Z. Fan, Lancet Oncol., 2009, 10, 541–542 CrossRef.
- G. G. Stefanini and D. R. Holmes, N. Engl. J. Med., 2013, 368, 254–265 CrossRef CAS PubMed.
- S.-R. Guo, Z.-M. Wang, Y.-Q. Zhang, L. Lei, J.-M. Shi, K.-M. Chen and Z. Yu, J. Pharm. Sci., 2010, 99, 3009–3018 CrossRef CAS PubMed.
- T. J. Song, S. S. Lee, S. C. Yun, D. H. Park, D. W. Seo, S. K. Lee and M.-H. Kim, Gastrointest. Endosc., 2011, 73, 727–733 CrossRef PubMed.
- E.-Y. Kim, H.-Y. Song, J. H. Kim, Y. Fan, S. Park, D.-K. Kim, E. W. Lee and H. K. Na, Radiology, 2013, 267, 396–404 CrossRef PubMed.
- S. R. Jeon, S. H. Eun, C. S. Shim, C. B. Ryu, J. O. Kim, J. Y. Cho, J. S. Lee, M. S. Lee and S. Y. Jin, Endoscopy, 2009, 41, 449–456 CrossRef CAS PubMed.
- S. Witold, M. Annick, H. Shunichi, Y. L'Hocine and R. Jean, Biomed. Mater., 2007, 2, S23 CrossRef PubMed.
- J. Wu and C.-C. Chu, J. Mater. Chem. B, 2013, 1, 353–360 RSC.
- L. V. Karabanova, S. V. Mikhalovsky and A. W. Lloyd, J. Mater. Chem., 2012, 22, 7919–7928 RSC.
- J. Y. Cherng, T. Y. Hou, M. F. Shih, H. Talsma and W. E. Hennink, Int. J. Pharm., 2013, 450, 145–162 CrossRef CAS PubMed.
- Z. Zhang, L. Mei and S.-S. Feng, Expert Opin. Drug Delivery, 2013, 10, 325–340 CrossRef CAS PubMed.
- A. B. Dhanikula and R. Panchagnula, Int. J. Pharm., 1999, 183, 85–100 CrossRef CAS.
- N. L. Elstad and K. D. Fowers, Adv. Drug Delivery Rev., 2009, 61, 785–794 CrossRef CAS PubMed.
- J. E. Puskas, L. G. Muñoz-Robledo, R. A. Hoerr, J. Foley, S. P. Schmidt, M. Evancho-Chapman, J. Dong, C. Frethem and G. Haugstad, Wiley Interdiscip. Rev.: Nanomed. Nanobiotechnol., 2009, 1, 451–462 CrossRef CAS PubMed.
- N. F. Huang, S. Patel, R. G. Thakar, J. Wu, B. S. Hsiao, B. Chu, R. J. Lee and S. Li, Nano Lett., 2006, 6, 537–542 CrossRef CAS PubMed.
- W. Hwang, G. Xin, M. Cho, S. Cho and H. Chae, Nanoscale Res. Lett., 2012, 7, 1–7 CrossRef PubMed.
- C. J. Luo, S. D. Stoyanov, E. Stride, E. Pelan and M. Edirisinghe, Chem. Soc. Rev., 2012, 41, 4708–4735 RSC.
- L. T. de Jonge, S. C. G. Leeuwenburgh, J. J. J. P. van den Beucken, J. G. C. Wolke and J. A. Jansen, Adv. Funct. Mater., 2009, 19, 755–762 CrossRef CAS.
- J.-H. Guo, G.-J. Teng, G.-Y. Zhu, S.-C. He, G. Deng and J. He, Eur. J. Radiol., 2007, 61, 356–361 CrossRef PubMed.
- P. Didden, M. C. W. Spaander, M. J. Bruno and E. J. Kuipers, Curr. Gastroenterol. Rep., 2013, 15, 1–9 Search PubMed.
- S. Spechler, JAMA, J. Am. Med. Assoc., 2013, 310, 627–636 CrossRef CAS PubMed.
- A. S. Ross and R. A. Kozarek, Gastrointest. Endosc. Clin., 2011, 21, 535–545 CrossRef PubMed.
- K. S. Dua, Gastrointest. Endosc. Clin., 2011, 21, 359–376 CrossRef PubMed.
- H. Y. Song, S. I. Park, Y. S. Do, H. K. Yoon, K. B. Sung, K. H. Sohn and Y. I. Min, Radiology, 1997, 203, 131–136 CrossRef CAS PubMed.
- S. Garg, C. Bourantas and P. W. Serruys, Nat. Rev. Cardiol., 2013, 10, 248–260 CrossRef CAS PubMed.
- L. Zhao, J. Wu, H. Zhou, A. Yuan, X. Zhang, F. Xu and Y. Hu, Curr. Gene Ther., 2011, 11, 423–432 CrossRef CAS.
- K. Kojio, S. Kugumiya, Y. Uchiba, Y. Nishino and M. Furukawa, Polym. J., 2008, 41, 118–124 CrossRef.
- E. Delebecq, J.-P. Pascault, B. Boutevin and F. Ganachaud, Chem. Rev., 2012, 113, 80–118 CrossRef PubMed.
- E. Iofina, R. Langenberg, R. Blindt, H. Kühl, M. Kelm and R. Hoffmann, Am. J. Cardiol., 2006, 98, 1022–1027 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra01300j |
| ‡ Lili Zhao and Ying Gao contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2014 |