Superior cycling and rate performances of rattle-type CoMoO4 microspheres prepared by one-pot spray pyrolysis†
You Na Koab,
Yun Chan Kang*a and
Seung Bin Parkb
aDepartment of Chemical Engineering, Konkuk University, 1 Hwayang-dong, Gwangjin-gu, Seoul 143-701, Korea. E-mail: yckang@konkuk.ac.kr; Fax: +82-2-458-3504; Tel: +82-2-2049-6010
bDepartment of Chemical and Biomolecular Engineering, Korea Advanced Institute of Science and Technology, 291 Daehak-ro, Yuseong-gu, Daejeon 305-701, Korea
First published on 4th April 2014
Abstract
Rattle-type CoMoO4 and CoMoO4–carbon composite microspheres were prepared by one-pot spray pyrolysis at temperatures of 850 and 700 °C, respectively. The XRD patterns of both the samples corresponded to the pure crystal structure of β-CoMoO4. The CoMoO4–carbon composite microspheres exhibited broad diffraction peaks with relatively lower intensities, when compared to those of rattle-type CoMoO4 microspheres. This indicates the poor crystallinity of the carbon composite powders, despite the similar preparation conditions. In the initial cycles, the rattle-type CoMoO4 microspheres and CoMoO4–carbon composite microspheres delivered discharge capacities of 1221 and 1245 mA h g−1, respectively at a current density of 500 mA g−1, and charge capacities of 1019 and 896 mA h g−1, respectively, corresponding to Coulombic efficiencies of 83 and 72%, respectively. After 150 cycles, the discharge capacities of the rattle-type and carbon composite microspheres were 1065 and 833 mA h g−1, respectively, and the corresponding capacity retentions measured after the first cycles were 100 and 90%, respectively. The morphology of the rattle-type CoMoO4 microsphere was maintained, despite repeated Li+ insertion and extraction processes, even at a high current density of 500 mA g−1.
Introduction
Recently, a variety of transition metal oxides have been actively investigated as electrode materials for lithium ion batteries (LIBs).1–5 However, the practical application of these materials have been greatly limited due to their inherent poor cycling stability resulting from the large volume change during Li+ insertion/extraction, and large initial irreversible capacities.6–8 In order to circumvent these limitations and improve the cycling stability, binary metal oxides have been proposed as anode materials.9–17 These materials can buffer the volume change by separating into single metal oxides during cycling.17 Nevertheless, the large initial irreversible capacity of the binary oxides still hinders their practical application. Therefore, significant research efforts have been devoted on searching new anode materials with stable cycling capacity and low irreversible capacity. Recently, AMoO4-type metal molybdates (where A is a divalent metal ion like Co, Ni, Fe, Zn, Ca, Mn, and so on) have emerged as prospective materials for energy storage.18–29 The AMoO4-type compounds are formed by the combination of molybdenum oxide and metal oxides, wherein the MoO3 offers the advantages of multiple oxidation states (from 3+ to 6+) and conversion reactions at low potential, suitable for use in LIBs.27–29 Among them, Co–Mo binary compound exhibits high reversible capacity and excellent cycling stability.29Thus far, several strategies have been proposed to improve the electrochemical performance, which not only include the exploration of new electrode materials but also the fabrication of electrode materials with controlled morphology.30–37 In principle, designing unique nanostructures is expected to provide more efficient charge storage sites, shorter Li+ ion and electron diffusion distance, and the ability to withstand volume change during cycling, thereby leading to improved rate performance and cycling stability. However, to the best of our knowledge, the improved rate and cycling performances of CoMoO4 as anode material for LIBs have rarely been reported.
In this study, we report the fabrication of rattle-type CoMoO4 microspheres by using a one-step spray pyrolysis process. The simple and effective spray pyrolysis process adopted in this study offered the feasibility to design CoMoO4 as a suitable anode material for LIBs. The well-designed CoMoO4 exhibited low initial irreversible capacity and good cycling stability at high current density.
Experimental
Synthesis of rattle-type CoMoO4 and CoMoO4–carbon composite microspheres
The rattle-type CoMoO4 and CoMoO4–carbon composite microspheres were prepared via one-pot spray pyrolysis process. Fig. S1† shows the schematic diagram of the ultrasonic spray pyrolysis system used for the synthesis of the CoMoO4 powders. In the typical system, the droplets were generated using a 1.7 MHz ultrasonic spray generator consisting of six vibrators. The droplets thus generated were then carried to a quartz reactor of length 1200 mm and diameter 50 mm. Air was used as the carrier gas, at a flow rate of 10 L min−1. The reactor temperature was maintained at 700 °C and 850 °C for the synthesis of CoMoO4–carbon composite microspheres and rattle-type CoMoO4 microspheres, respectively. The aqueous spray solution was prepared by dissolving 0.1 M of MoO3, 0.1 M of cobalt nitrate, and 0.7 M of sucrose in a mixture of hydrogen peroxide and distilled water under heating. Sucrose was used as the carbon source for the synthesis of CoMoO4–carbon composite powders. The formation mechanisms of CoMoO4–carbon composite microsphere and rattle-type CoMoO4 microsphere in the spray pyrolysis were described in Fig. S2.†Characterizations
The morphology of the CoMoO4 microspheres prepared by spray pyrolysis was observed by using scanning electron microscope (SEM, JEOL JSM-6060), and transmission electron microscope (FE-TEM, JEM-2100F). The crystal structures of the prepared powders were investigated by X-ray diffraction (XRD, X'Pert PRO MPD) using Cu Kα radiation (λ = 1.5418 Å) at the Korea Basic Science Institute (Daegu). The amount of carbon in the powders was determined by using thermal gravimetric analysis (TGA, SDT Q600) performed in air at a heating rate of 10 °C min−1. The specific surface area of the powders was measured by the Brunauer–Emmett–Teller (BET) method using N2 as the adsorbate gas.Electrochemical measurements
The electrochemical properties of the prepared powders were analyzed by fabricating a 2032-type coin cell. The anode was prepared from a mixture of the active material, carbon black, and sodium carboxymethyl cellulose (CMC) in a weight ratio of 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The size of the negative electrode with a thickness of 34 μm was 1 cm × 1 cm and the mass loading was 2 mg cm−2. Li metal and microporous polypropylene film were used as the counter electrode and separator, respectively. The electrolyte was composed of 1 M LiPF6 dissolved in a mixture of fluoroethylene carbonate–dimethyl carbonate (FEC/DMC; 1
1. The size of the negative electrode with a thickness of 34 μm was 1 cm × 1 cm and the mass loading was 2 mg cm−2. Li metal and microporous polypropylene film were used as the counter electrode and separator, respectively. The electrolyte was composed of 1 M LiPF6 dissolved in a mixture of fluoroethylene carbonate–dimethyl carbonate (FEC/DMC; 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v). The discharge–charge characteristics of the samples were investigated by cycling in the voltage range of 0.001–3 V at various current densities. The cyclic voltammograms were measured at a scan rate of 0.1 mV s−1.
1 v/v). The discharge–charge characteristics of the samples were investigated by cycling in the voltage range of 0.001–3 V at various current densities. The cyclic voltammograms were measured at a scan rate of 0.1 mV s−1.
Results and discussion
In this study, CoMoO4–carbon composite and rattle-type CoMoO4 microspheres were prepared through one-step spray pyrolysis at temperatures of 700 and 850 °C, respectively. The crystal structure of the CoMoO4 powders, as determined from the XRD pattern shown in Fig. 1a, is in good agreement with the β-CoMoO4 phase (JCPDS card no. 21-0868). In addition, the XRD pattern of the rattle-type CoMoO4 microspheres exhibited sharp diffraction peak, indicating the high crystallinity of the sample. The mean crystallite size of the rattle-type CoMoO4 microspheres measured by Scherrer's equation from the peak width of the XRD pattern was 58 nm. On the other hand, the CoMoO4–carbon composite microspheres exhibited broad peaks with relatively lower intensities compared to those of rattle-type CoMoO4 microspheres, indicating the poor crystallinity of the carbon composite powders despite the similar preparation conditions. This could be attributed to that the carbon component restricts the growth of CoMoO4 phase during the spray pyrolysis process. | ||
| Fig. 1 (a) XRD patterns and (b) TG curves of rattle-type CoMoO4 microspheres and CoMoO4–carbon composite microspheres. | ||
Furthermore, the amount of carbon in the prepared samples was analyzed by thermal gravimetric (TG) analysis in the temperature range of 20–800 °C, under flowing air atmosphere (Fig. 1b). As can be seen from the TG curve, the CoMoO4–carbon composite microspheres display three weight loss steps. The first weight loss at temperature below 200 °C can be attributed to the loss of water molecules adsorbed on the composite powders. The weight loss corresponding to the oxidation of carbon occurred via two steps. From the TG curve, the carbon content in the CoMoO4–carbon composite powder was estimated to be 26.1 wt%. In contrast, weight loss was rarely observed in the TG curve of the rattle-type CoMoO4 microspheres. The carbon component was completely decomposed during the formation of the rattle-type CoMoO4 microspheres at a relatively high temperature of 850 °C. In other words, the composition of carbon component of the CoMoO4–carbon composite formed as an intermediate produced the rattle-type CoMoO4 microspheres.
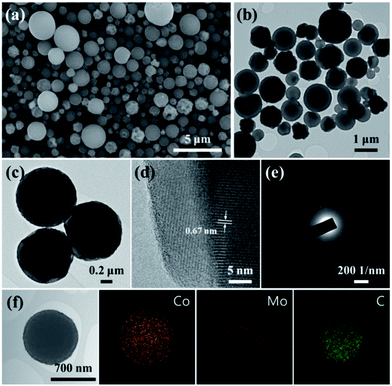
Fig. 2 shows the morphology of the rattle-type CoMoO4 microspheres, as observed by using SEM and TEM. Fig. 2a reveals that the CoMoO4 powders fabricated by one-step spray pyrolysis had a spherical shape, regardless of the size. The mean size of the rattle-type microspheres was estimated to be 0.9 μm. The TEM images shown in Fig. 2b and c clearly indicate that the CoMoO4 microspheres have a rattle-type structure composed of a porous shell and a solid core, with hollow space between the core and shell. As can be seen from the Fig. 2c, the rattle-type microspheres are composed of aggregated nanocrystals of CoMoO4. The mean grain size of the rattle-type microspheres measured from the TEM image as shown in Fig. 2c was about 80 nm. The high-resolution TEM (HRTEM) image and selected area electron diffraction (SAED) pattern shown in Fig. 2d and e indicate the high crystallinity of the rattle-type CoMoO4 microspheres. The HRTEM image displays clear lattice fringes with spacing of 0.67 nm, which close to the (001) plane of the monoclinic CoMoO4. The elemental-mapping images of the rattle-type CoMoO4 microspheres confirms the uniform dispersion of cobalt and molybdenum components, and the carbon component could be barely detected in the sample. The BET surface area of the rattle-type CoMoO4 microspheres was estimated to be 3.7 m2 g−1.
 | ||
| Fig. 2 Morphologies of rattle-type CoMoO4 microspheres: (a) SEM image, (b) and (c) TEM image, (d) high-resolution TEM image, (e) SAED pattern, and (f) elemental-mapping images. | ||
Fig. 3 shows the morphology of the CoMoO4–carbon composite powder synthesized by spray pyrolysis at 700 °C. The SEM image of the composite powder shown in Fig. 3a indicates a spherical morphology. The mean size of the CoMoO4–carbon composite microspheres was estimated to be 1.0 μm, similar to that of the rattle-type CoMoO4 microspheres. The TEM images of the composite powder shown in Fig. 3b and c indicate a filled structure. The HRTEM of the boundary of the sample (Fig. 3d) shows distinct lattice fringes with distance of 0.67 nm, corresponding to the (001) plane of monoclinic CoMoO4. This is due to the combustion of the carbon component at the surface of the powders during the spray pyrolysis process. Even though, some powders had well-faceted crystals on the surface, the SAED pattern shown in Fig. 3e revealed poor crystallinity of the CoMoO4–carbon composite microspheres. The uniform distribution of each element can be observed from the elemental-mapping images shown in Fig. 3f. The formation mechanisms of CoMoO4–carbon composite microsphere and rattle-type CoMoO4 microsphere in the spray pyrolysis are described in Fig. S2.† The nondecomposition of amorphous carbon formed by polymerization and carbonization processes of sucrose resulted in the CoMoO4–carbon composite microsphere at a low preparation temperature of 700 °C. On the other hand, decomposition of carbon in the CoMoO4–carbon composite formed as an intermediate product in the front part of the reactor maintained at 850 °C resulted in the rattle-type CoMoO4 microsphere.38 The composite microspheres exhibited a large BET surface area of 48.3 m2 g−1, as compared with that of rattle-type microspheres (Fig. S3†) owing to the small crystalline particles and residual carbon components in the sample.
 | ||
| Fig. 3 Morphologies of CoMoO4–carbon composite microspheres: (a) SEM image, (b) and (c) TEM image, (d) high-resolution TEM image, (e) SAED pattern, and (f) elemental-mapping images. | ||
The electrochemical performances of the rattle-type CoMoO4 microspheres were compared with those of the CoMoO4–carbon composite microspheres (Fig. 4), in the voltage range of 0.001–3 V vs. Li/Li+. Fig. 4a shows the first five charge–discharge cycles in the cyclic voltammograms (CVs) of the rattle-type CoMoO4 microspheres. In the first discharge cycle, the reduction peak at around 0.4 V can be attributed to the reduction of CoMoO4 to form metallic nanograins of Co0 and Mo0.28,39,40 This reduction peak was not observed in the subsequent cycles, indicating the irreversible destruction of CoMoO4 structure. In the subsequent cycles, three cathodic peaks emerged at around 1.54, 0.6, and 0.16 V, which could be related to the sequential insertion of Li+ ions into CoO and MoO3.28 In the first charge cycle, two anodic peaks were observed at around 1.45 and 1.8 V. The first anodic peak at 1.45 V is ascribed to the oxidation of metallic Mo to Mo4+, while the second anodic peak at 1.8 V is ascribed to the oxidation of metallic Co to Co2+ and Mo4+ to Mo6+.28,39,40 Starting from the second cycle, the CV curves overlapped substantially, indicating the good cycling stability of the rattle-type CoMoO4 microspheres. Fig. 4b shows the initial discharge and charge curves of the rattle-type CoMoO4 microspheres and CoMoO4–carbon composite microspheres at a constant current density of 500 mA g−1. The voltage profile of the rattle-type microspheres and carbon composite microspheres showed a difference in the voltage separation, although the shape of the voltage profiles was almost similar. The voltage separation observed for the CoMoO4–carbon composite microspheres was smaller than that of the rattle-type CoMoO4 microspheres, indicating a lower electrode polarization originating due to the small primary particle size.40–42 In the initial cycle, the rattle-type CoMoO4 microspheres and CoMoO4–carbon composite microspheres delivered discharge capacities of 1221 and 1245 mA h g−1, respectively, and charge capacities of 1019 and 896 mA h g−1, respectively, corresponding to the Coulombic efficiency of 83 and 72%, respectively. The CoMoO4–carbon composite microspheres exhibited significantly large irreversible capacity in the initial cycle, compared to that of rattle-type CoMoO4 microspheres. This could be due to the large surface area of the composite that is available for side reaction and the large irreversibility caused by limited extraction of Li+ ions from amorphous carbon.43
Furthermore, the cycling performances of the rattle-type CoMoO4 microspheres were compared with that of CoMoO4–carbon composite microspheres, at a constant current density of 500 mA g−1 (Fig. 4c). After 150 cycles, the discharge capacities of the rattle-type and carbon composite microspheres were 1065 and 833 mA h g−1, respectively, and the corresponding capacity retentions measured after the first cycles were 100 and 90%. In addition, we compared the rate performances of the rattle-type and carbon composite microspheres, in which the current densities were increased stepwise from 300 to 1500 mA g−1 in the voltage range of 0.001–3 V (Fig. 4d). The rattle-type CoMoO4 microspheres exhibited high 10th cycle capacities of 1052, 1000, 946, 871, and 783 mA h g−1 at current densities of 300, 600, 900, 1200, and 1500 mA g−1, respectively. Overall, the rattle-type CoMoO4 microspheres demonstrated better rate performance than that of the CoMoO4–carbon composite microspheres. Even at a high current density of 1500 mA g−1, the discharge capacity of the rattle-type CoMoO4 microspheres was as high as that of the CoMoO4–carbon composite microspheres at a low current density.
Fig. 4e and S6† show the morphologies of the rattle-type CoMoO4 microspheres and CoMoO4–carbon composite microspheres after 150 charge–discharge cycles. The rattle-type CoMoO4 microspheres and CoMoO4–carbon composite microspheres maintained their spherical morphology, despite repeated Li+ insertion and extraction processes, even at a high current density of 500 mA g−1. Fig. 4f illustrates the structural changes in the rattle-type microspheres during Li+ insertion and extraction. The rattle-type structure of CoMoO4 facilitates fast kinetics of Li+ ions and electrons, and buffers the volume change during cycling. In other terms, controlling the morphology of CoMoO4 in the form of a rattle-type structure results in superior electrochemical performances with low irreversible capacity, high capacity, good cycling, and structural stabilities at high current densities, making them promising anode materials for LIBs.
Conclusions
In summary, we have compared the electrochemical properties of rattle-type CoMoO4 microspheres and CoMoO4–carbon composite microspheres prepared by a facile one-pot spray pyrolysis process. The CoMoO4–carbon composite microspheres were prepared by spray pyrolysis at a relatively low temperature of 700 °C, whereas the rattle-type CoMoO4 microspheres were prepared at a temperature of 850 °C. It is believed that the combustion of carbon component in the CoMoO4–carbon composite intermediate at the preparation temperature of 850 °C resulted in the formation of rattle-type CoMoO4 microspheres. The rattle-type CoMoO4 microspheres had higher discharge and charge capacities and Coulombic efficiency, when compared to those of the CoMoO4–carbon composite microspheres in the first cycles. The discharge capacities of the rattle-type CoMoO4 microspheres for the 2nd and 150th cycles at a current density of 500 mA g−1 were 1063 and 1065 mA h g−1, respectively. The rattle-type CoMoO4 microspheres had high structural stability and hence had good rate performance. These results suggest that, controlling the morphology of CoMoO4 in the form of a rattle-type structure resulted in superior electrochemical performances compared to those of the CoMoO4–carbon composite microspheres.Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MEST) (no. 2012R1A2A2A02046367). This work was supported by the Converging Research Center Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2011-50210). This work was supported by the Energy Efficiency & Resources Core Technology Program of the Korea Institute of Energy Technology Evaluation and Planning (KETEP), granted financial resource from the Ministry of Trade, Industry & Energy, Republic of Korea (201320200000420).Notes and references
- P. Poizot, S. Laruelle, S. Grugeon, L. Dupont and J. M. Tarascon, Nature, 2000, 407, 496–499 CrossRef CAS PubMed
.
- M. V. Reddy, G. V. Subba Rao and B. V. R. Chowdari, Chem. Rev., 2013, 113, 5364–5457 CrossRef CAS PubMed
.
- P. Poizot, S. Laruelle, S. Grugeon, L. Dupont and J. M. Tarascon, J. Power Sources, 2001, 97–98, 235–239 CrossRef CAS
.
- A. L. M. Reddy, M. M. Shaijumon, S. R. Gowda and P. M. Ajayan, Nano Lett., 2009, 9, 1002–1006 CrossRef CAS PubMed
.
- X. W. Lou, D. Deng, J. Yang Lee, J. Feng and L. A. Archer, Adv. Mater., 2008, 20, 258–262 CrossRef CAS
.
- G. Zhou, D. W. Wang, F. Li, L. Zhang, N. Li, Z. S. Wu, L. Wen, G. Q. Lu and H. M. Cheng, Chem. Mater., 2010, 22, 5306–5313 CrossRef CAS
.
- S. Yang, Y. Sun, L. Chen, Y. Hernandez, X. Feng and K. Müllen, Sci. Rep., 2012, 2, 427 Search PubMed
.
- X. Zhu, Y. Zhu, S. Murali, M. D. Stoller and R. S. Ruoff, ACS Nano, 2011, 5, 3333–3338 CrossRef CAS PubMed
.
- D. Wadewitz, W. Gruner, M. Herklotz, M. Klose, L. Giebeler, A. Voß, J. Thomas, T. Gemming, J. Eckert and H. Ehrenberg, J. Electrochem. Soc., 2013, 160, A1333–A1339 CrossRef CAS PubMed
.
- F. M. Courtel, H. Duncan, Y. Abu-Lebdeh and I. J. Davidson, J. Mater. Chem., 2011, 21, 10206–10218 RSC
.
- M. Li, Y. X. Yin, C. Li, F. Zhang, L. J. Wan, S. Xu and D. G. Evansa, Chem. Commun., 2012, 48, 410–412 RSC
.
- L. Yu, L. Zhang, H. B. Wu, G. Zhang and X. W. Lou, Energy Environ. Sci., 2013, 6, 2664–2671 CAS
.
- J. Li, S. Xiong, Y. Liu, Z. Ju and Y. Qian, ACS Appl. Mater. Interfaces, 2013, 5, 981–988 CAS
.
- S. H. Choi and Y. C. Kang, ChemSusChem, 2013, 6, 2111–2116 CrossRef CAS PubMed
.
- J. G. Kim, S. H. Lee, Y. Kim and W. B. Kim, ACS Appl. Mater. Interfaces, 2013, 5, 11321–11328 CAS
.
- G. Zhang, L. Yu, H. B. Wu, H. E. Hoster and X. W. Lou, Adv. Mater., 2012, 24, 4609–4613 CrossRef CAS PubMed
.
- Y. Sharma, N. Sharma, G. V. Subba Rao and B. V. R. Chowdari, Adv. Funct. Mater., 2007, 17, 2855–2861 CrossRef CAS
.
- L. Q. Mai, F. Yang, Y. L. Zhao, X. Xu, L. Xu and Y. Z. Luo, Nat. Commun., 2012, 2, 381–385 CrossRef PubMed
.
- M. C. Liu, L. B. Kong, C. Lu, X. J. Ma, X. M. Li, Y. C. Luo and L. Kang, J. Mater. Chem. A, 2013, 1, 1380–1387 CAS
.
- D. Guo, H. Zhang, X. Yu, M. Zhang, P. Zhang, Q. Li and T. Wang, J. Mater. Chem. A, 2013, 1, 7247–7254 CAS
.
- Z. Xu, Z. Li, X. Tan, C. M. B. Holt, L. Zhang, B. S. Amirkhiz and D. Mitlin, RSC Adv., 2012, 2, 2753–2755 RSC
.
- B. Senthilkumar, K. V. Sankar, R. K. Selvan, M. Danielle and M. Manickam, RSC Adv., 2013, 3, 352–357 RSC
.
- N. N. Leyzerovich, K. G. Bramnik, T. Buhrmester, H. Ehrenberg and H. Fuess, J. Power Sources, 2004, 127, 76–84 CrossRef CAS PubMed
.
- W. Xiao, J. S. Chen, C. M. Li, R. Xu and X. W. Lou, Chem. Mater., 2010, 22, 746–754 CrossRef CAS
.
- J. Haetge, C. Suchomski and T. Brezesinski, Small, 2013, 9, 2541–2544 CrossRef CAS PubMed
.
- J. Haetge, I. Djerdj and T. Brezesinski, Chem. Commun., 2012, 48, 6726–6728 RSC
.
- S. S. Kim, S. Ogura, H. Ikuta, Y. Uchimoto and M. Wakihara, Solid State Ionics, 2002, 146, 249–256 CrossRef CAS
.
- K. S. Park, S. D. Seo, H. W. Shim and D. W. Kim, Nanoscale Res. Lett., 2012, 7, 35 CrossRef PubMed
.
- C. T. Cherian, M. V. Reddy, S. C. Haur and B. V. R. Chowdari, ACS Appl. Mater. Interfaces, 2013, 5, 918–923 CAS
.
- M. D. Fleischauer and J. R. Dahn, J. Electrochem. Soc., 2004, 151, A1216–A1221 CrossRef CAS PubMed
.
- P. F. Teh, S. S. Pramana, Y. Sharma, Y. W. Ko and S. Madhavi, ACS Appl. Mater. Interfaces, 2013, 5, 5461–5467 CAS
.
- L. Ji, Z. Lin, M. Alcoutlabi and X. Zhang, Energy Environ. Sci., 2011, 4, 2682–2699 CAS
.
- M. G. Kim and J. Cho, Adv. Funct. Mater., 2009, 19, 1497–1514 CrossRef CAS
.
- Z. Wang, L. Zhou and X. W. Lou, Adv. Mater., 2012, 24, 1903–1911 CrossRef CAS
.
- W. Shi, X. Rui, J. Zhu and Q. Yan, J. Phys. Chem. C, 2012, 116, 26685–26693 CAS
.
- H. B. Wu, J. S. Chen, H. H. Hng and X. W. Lou, Nanoscale, 2012, 4, 2526–2542 RSC
.
- J. Ye, H. Zhang, R. Yang, X. Li and L. Qi, Small, 2010, 6, 296–306 CrossRef CAS PubMed
.
- Y. J. Hong, M. Y. Son and Y. C. Kang, Adv. Mater., 2013, 25, 2279–2283 CrossRef CAS PubMed
.
- Y. Sun, J. Wang, B. Zhao, R. Cai, R. Ran and Z. Shao, J. Mater. Chem. A, 2013, 1, 4736–4746 CAS
.
- Z. S. Wu, W. Ren, L. Wen, L. Gao, J. Zhao, Z. Chen, G. Zhou, F. Li and H. M. Cheng, ACS Nano, 2010, 4, 3187–3194 CrossRef CAS PubMed
.
- V. Subramanian, A. Karki, K. I. Gnanasekar, F. P. Eddy and B. Rambabu, J. Power Sources, 2006, 159, 186–192 CrossRef CAS PubMed
.
- M. T. McDowell, S. W. Lee, I. Ryu, H. Wu, W. D. Nix, J. W. Choi and Y. Cui, Nano Lett., 2011, 11, 4018–4025 CrossRef CAS PubMed
.
- Y. S. Hu, P. Adelhelm, B. M. Smarsly, S. Hore, M. Antonietti and J. Maier, Adv. Funct. Mater., 2007, 17, 1873–1878 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra01278j |
| This journal is © The Royal Society of Chemistry 2014 |

