A catalase-magnetic switch for cell proliferation†
Abstract
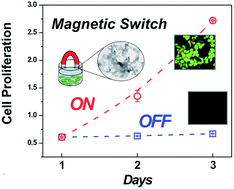
The advance of novel biotechnology requires the development of versatile control systems for cell behavior. Towards the vision of cell growth control, we report a magnetic switching in cell proliferation by a catalase–nanomagnetite complex. The system synergically exploits both the ability of catalase to scavenge cell-generated hydrogen peroxide, a messenger involved in cell cycle regulation, and magnetite manipulation to modulate cell proliferation between arrest and growth using magnetic fields. This ON/OFF switch methodology could constitute the basis of new classes of diagnostic and therapeutic strategies as well as novel integrated responsive biomaterials.

 Please wait while we load your content...
Please wait while we load your content...